Clinical and preclinical tolerance protocols for vascularized composite allograft transplantation
Article information
Abstract
The field of vascularized composite allografts (VCAs) has undergone significant advancement in recent decades, and VCAs are increasingly common and accepted in the clinical setting, bringing hope of functional recovery to patients with debilitating injuries. A major obstacle facing the widespread application of VCAs is the side effect profile associated with the current immunosuppressive regimen, which can cause a wide array of complications such as infection, malignancy, and even death. Significant concerns remain regarding whether the treatment outweighs the risk. The potential solution to this dilemma would be achieving VCA tolerance, which would allow recipients to receive allografts without significant immunosuppression and its sequelae. Promising tolerance protocols are being studied in kidney transplantation; four major trials have attempted to withdraw immunosuppressive treatment with various successes. The common theme in all four trials is the use of radiation treatment and donor cell transplantation. The knowledge gained from these trials can provide valuable insight into the development of a VCA tolerance protocol. Despite similarities, VCAs present additional barriers compared to kidney allografts regarding tolerance induction. VCA donors are likely to be deceased, which limits the time for significant pre-conditioning. VCA donors are also more likely to be human leukocyte antigen–mismatched, which means that tolerance must be induced across major immunological barriers. This review also explores adjunct therapies studied in large animal models that could be the missing element in establishing a safe and stable tolerance induction method.
INTRODUCTION
Vascularized composite allograft (VCA) transplantation can restore significant structural and functional deficits while providing a socially acceptable appearance for patients who have suffered from severe tissue loss and diminished quality of life. Despite the complexity of both technical and immunological aspects of VCA transplantation, advancements in the field have resulted in over 100 cases of hand transplantations and over 40 cases of facial transplantations [1,2]. Other reported VCA transplantations include those of the flexor tendon apparatus, knee, larynx, uterus, and penis [3]. The field has also come a long way in the eyes of research administrators. A survey study targeting institutional review boards revealed that most members believed that VCA transplantation would require institutional review board approval only if done in conjunction with a research study [4]. Despite these achievements, many obstacles remain before the widespread application of VCAs. The most daunting roadblock is the need for lifelong immunosuppression (IS) and the associated sequelae.
The complications of IS for solid organ transplantation (SOT) are well studied. These complications include rejection, an increased risk of chronic renal disease, diabetes, infection, osteonecrosis, and malignancy [5-7]. In functional kidney transplantation patients, the transplantation-related death rate can be as high as 25% after 10 years of treatment [8]. In addition to the technical complexity of VCA transplantations due to the immunogenicity of the skin, VCA patients may require even higher doses of IS therapy than those used in patients undergoing SOT [9]. Therefore, some controversy surrounds VCA transplantation, making otherwise healthy patients vulnerable to similar complications with SOT patients.
Approximately 85% of VCA recipients can experience one or more acute rejection episodes during their first year of transplantation [10]. These episodes are taxing on patients and require high-dose steroid treatment or increased doses of other IS medications [11,12]. Even after acute rejection episodes, chronic rejection (CR) may still develop in many patients [13-15]. Although CR can potentially be kept at bay with consistent IS, a decreased dose may be inevitable due to the development of intolerable or life-threatening side effects, as evident in the literature [14].
The financial burden of IS is also a significant barrier, with treatment potentially costing $14,000 annually for kidney transplant patients [16]. The cost poses an ethical dilemma for patients, clinicians, and society. Who should be allowed to have VCA? Who needs it the most? Is the need justified concerning the potential risk and side effects and the cost to the healthcare system? These concerns highlight the crucial need for a successful tolerance protocol that would allow acceptance of all components of a VCA without the need for high-dose chronic IS.
Allograft tolerance would allow for prolonged survival of the graft without acute or CR episodes and without the need for lifelong IS medications. With a reduction of IS drugs, there would be a substantial decrease in the overall complication rate. Four clinical trials performed at Massachusetts General Hospital, Stanford, Northwestern/Duke, and Samsung Medical Center aimed to achieve kidney allograft tolerance in human leukocyte antigen (HLA)–mismatched transplantations. Complete IS withdrawal has been attempted in these trials [17]. However, tolerance is a fragile state, and stability has yet to be achieved. In efforts to attain donor-specific tolerance, VCA preclinical research focuses on tolerance-inducing treatments, including costimulatory blockade, hematopoietic cell transplantation (HCT), cell-based therapy, and other novel approaches [18].
This review examines the current clinical and preclinical studies aimed at developing an effective tolerance strategy. We review the various groups with the most promising clinical tolerance induction protocols in kidney transplantation. We also analyze translational studies on large animals that hold promise for human trials in the future.
METHODS
This narrative review was mapped out by the senior author (DWM) to include relevant areas for the review. Following this outline, JHY and ACJ conducted searches in the PubMed, MEDLINE, and Embase databases. The search strategies included but were not limited to the following keywords: VCA tolerance, VCA rejection, and kidney allograft tolerance. Only studies published in English were considered. No limitations were placed on the time of publication. From the search results, titles and abstracts were reviewed by the authors to determine whether an in-depth review was needed. Disagreements on study inclusion were brought to and settled by the senior author.
CURRENT VCA REGIMENS AND LIMITATIONS
In the first facial transplantation conducted by the Devauchelle group in France, the IS regimen utilized rabbit anti-thymocyte globulin induction (rATG), followed by tacrolimus and mycophenolate mofetil (MMF), with prednisone tapering to a lower dosage. For general infection prevention, amoxicillin-clavulanate was administered. For cytomegalovirus prophylaxis, patients received intravenous ganciclovir followed by valganciclovir. Trimethoprim-sulfamethoxazole was used for Pneumocystis jiroveci pneumonia prevention. Donor nucleated hematopoietic cells were also infused on post-transplant days 4 and 11 [19].
Clinical VCA IS protocols have remained largely similar to established SOT IS protocols, although many groups have introduced modifications intended to reduce side effects and toxicity. Different induction agents have been used, such as humanized IL2 receptor antibody [20,21], alemtuzumab [22], and rituximab [23]. Diaz-Siso et al. [24] worked on steroid-free dual maintenance treatment through gradual weaning of steroids and continuation of tacrolimus and MMF. The Pittsburgh group has used alemtuzumab and methylprednisolone induction followed by donor bone marrow infusion and maintenance on tacrolimus monotherapy in upper extremity transplantation [25]. Grahammer et al. [26] introduced belatacept, a T cell inhibitor, as a maintenance agent in hand transplantation to reduce the tacrolimus target level. A report of graft loss due to vasculopathy in a hand transplantation patient on a steroid-sparing protocol has raised caution regarding withdrawing steroids from treatment [27]. Given the small number of VCA recipients undergoing treatment, it is difficult to complete an adequately powered study to compare different IS regimens at this time.
Acute rejection treatment
Rejections frequently occur in VCA recipients, Hautz et al. [28] recorded a total of 43 rejection episodes in five hand and forearm transplantation patients. Out of the 43 episodes, the majority were T-cell mediated rejections, along with 12 antibody-mediated rejections, one B-cell mediated rejection, and one CR. Acute rejection is well defined in VCA, and assessment via the Banff criteria has been developed and applied to the skin [29]. When acute rejection occurs, a steroid bolus is often the firstline medication used for treatment. Guo et al. [20] reported three acute rejection incidents at 3, 7, and 17 months after facial transplantation. Treatment included increasing tacrolimus dosage from 15 ng/mL to 25 ng/mL and methylprednisolone pulse therapy (1 g, 0.5 g, 0.5 g, 0.25 g, 0.125 g) for the first 5 days, followed by prednisolone starting at 80 mg daily tapering eventually to 15 mg daily as a long-term maintenance dose. Pomahac et al. [30] reported two cases of acute rejection, both successfully treated with pulse doses of methylprednisolone. Basiliximab use has also been reported in acute rejection, along with alemtuzumab reported effective in a steroid-resistant episode [31]. Barret et al. [32] treated acute rejection with 1.5 g/kg of rATG and replacing MMF with sirolimus. It is worth noting that hyperacute rejection, although established in SOT, rarely occurs in VCA setting and is therefore not well studied [29,33].
Chronic rejection
A significant challenge in discussing CR in VCA transplantation is the lack of an official definition due to the limited number of cases and short follow-up time. Another challenge is that the manifestation of CR in VCA may differ from what is known in SOT, potentially requiring the establishment of new sets of criteria [13]. In the first case of human face transplant, the patient developed CR of the graft with histologic evidence of intimal thickening, thrombosis of pedicle vessels, and C4d deposits on the endothelium of dermal vessels. The antibody-mediated rejection eventually led to surgical removal of the lower lip, labial commissures, and right cheek [34]. Petruzzo et al. [14] reported a case of CR first noticed in the skin, with skin sclerosis at around year 2 after scheduled IS reduction due to Epstein-Barr virus-related tumor complications. Overall, the pathogenic mechanism of CR in VCA requires further research.
Mortality
Death is a feared complication of any medical procedure, particularly concerning when it occurs due to non-life-threatening treatment such as VCA transplantation. Cases of VCA-associated mortality have been reported worldwide. The first reported mortality occurred in facial VCA transplantation patients, likely due to medical non-adherence [35]. Multiple other deaths in VCA recipients have been reported since then [29]. It is difficult to estimate the mortality rate associated with VCA due to caseby-case reporting. It would be highly beneficial for the understanding of VCA morbidity and mortality if a centralized database and reporting procedure can be adopted.
VCA monitoring
Multiple studies have assessed biomarkers of VCA rejection. Lian et al. [36] found a significant fluctuation in FoxP3:CD8 ratio that decreased with rejection episodes and reverted to prerejection levels following therapy. Kollar et al. [37] found in a pilot study that significantly elevated serum matrix metalloproteinase three levels, as determined by enzyme-linked immunosorbent assay, were correlated with severe rejection in facial transplantation patients. Despite these findings, a recent review by Honeyman et al. [38] outlined the need for a biomarker that can identify the very early stages of rejection. An assessment of VCA transplantation rejection via skin or sentinel flap is one clinical strategy for tolerance monitoring. An earlier approach in this area involves utilizing skin grafts in hand transplantation. However, the skin graft was observed to undergo different immune responses than VCAs, and this practice was discontinued [39]. A potentially more effective strategy is to used vascularized sentinel flaps at a discreet location such as the inframammary fold, with one report of excellent concordance rate between the sentinel flap and the facial allograft [40].
CLINICAL PROTOCOLS FOR KIDNEY ALLOGRAFT TOLERANCE
Massachusetts General Hospital
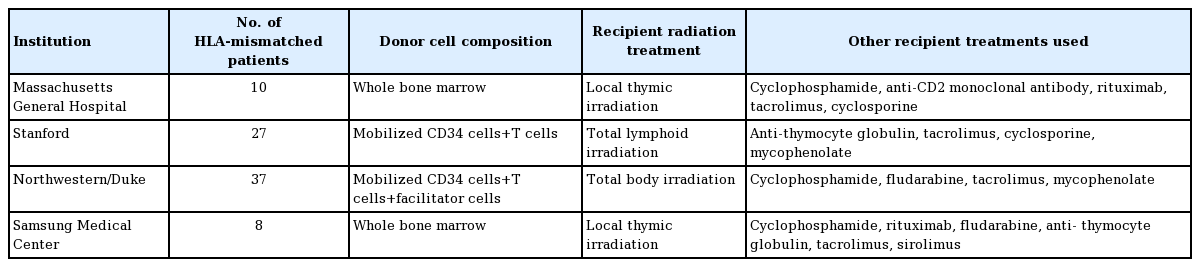
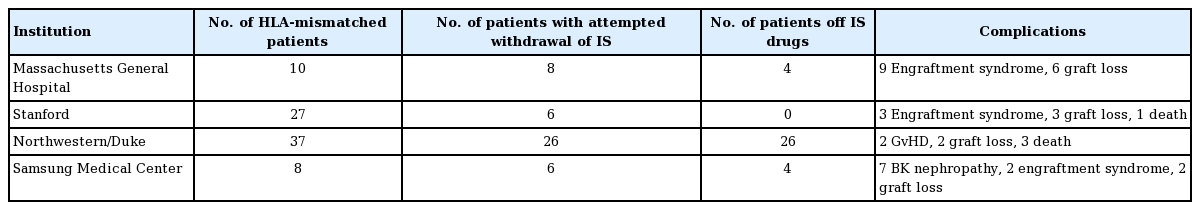
The Massachusetts General Hospital group conducted studies in 10 HLA haplotype-mismatched kidney transplant recipients. The conditioning regimen initially consisted of pre-transplant cyclophosphamide, humanized anti-CD2 monoclonal antibody, and local thymic irradiation, followed by a post-transplantation regimen of intravenous donor bone marrow cells (BMCs) infusion with calcineurin inhibitors (CNI) tapering starting at 6 months and complete discontinuation at 9–14 months [41]. Due to the strong humoral response, some patients also received peritransplant rituximab injection. All 10 patients achieved transient mixed chimerism for up to 3 weeks without signs of graft-versus-host disease (GvHD) [42]. Seven out of 10 patients had IS-free renal allograft survival for more than 5 years. Four of these patients had remained IS-free for more than 10 years, while three recipients lost graft function at around 10 years [17,43]. Due to acute kidney injury observed in nine out of 10 recipients, an additional pilot trial was conducted in two patients, replacing cyclophosphamide with low-dose total body irradiation (TBI). One patient had IS-free normal kidney function for 3.75 years, while the other patient failed to develop chimerism. Another pilot trial was conducted using a similar regimen except for rATG and belatacept replacing anti-CD2 mAB and low-dose TBI replacing cyclophosphamide. Both patients failed to develop sufficient chimerism and IS was never withdrawn (Tables 1, 2) [17].
Stanford
The Stanford group aimed to establish tolerance through persistent mixed chimerism, which has reduced GvHD and immunodeficiency risk compared to full donor chimerism. IS withdrawal was successful in many HLA-matched patients who developed chimerism. No IS withdrawals were successful in the HLA-mismatched patients. The protocol consists of combined kidney and HCT from the same donor. Fifty-six patients were enrolled, with 29 patients fully matched and 27 patients haplotypematched. Both groups received similar conditioning protocols, except for the haplotype-matched groups receiving a higher number of T cells. For donors, progenitor cells were collected by apheresis after injection of granulocyte colony-stimulating factors. The recipients received 10 doses of total lymphoid irradiation and five rATG doses over 11 days after transplantation. After completing total lymphoid irradiation, the CD34+ hematopoietic progenitor cells and a defined number of T cells collected from donors were injected into the recipients. Patients were also maintained on MMF and CNI. In patients developing persistent mixed chimerism, MMF was withdrawn after 1 month, and CNI was withdrawn around 1 year. It is worth noting that none of the 56 patients developed severe or chronic infection or GvHD. In the fully matched patients, 23 out of 29 patients have been completely withdrawn from maintenance IS regimen without signs of rejection for up to 14 years. In the HLA haplotype-matched group, the same withdrawal was attempted and resulted in the loss of chimerism; subsequently, no patient in this group was withdrawn entirely from IS regimen. There has been one death associated with stroke and two deaths associated with pulmonary embolism and coronary artery disease in patients with normal graft function. Additionally, two graft losses were seen due to kidney disease relapse [44-46].
Northwestern/Duke
The Northwestern/Duke group aimed at inducing durable donor chimerism for tolerance. The study utilized combined kidney and hematopoietic cell transplants in HLA-mismatched living donors. The protocol was built upon tolerogenic CD8+/TCR-facilitating cells and nonmyeloablative conditioning. The regimen includes fludarabine, cyclophosphamide, TBI, and donor apheresis product processed to retain CD34+ cells and facilitating cells. Thirty-seven patients have been transplanted under this trial, all with greater than 3 years of follow-up. MMF and tacrolimus-based IS were tapered and discontinued by the 1-year mark if chimerism remained present along with normal kidney function and biopsy results. Durable chimerism allowing full IS withdrawal happened in 26 patients, and out of these, 23 showed > 95% donor whole blood and T cell chimerism. All stable chimeric patients after IS withdrawal retained their chimerism and remained rejection-free by the latest report. Two cases of GvHD occurred, with one associated death. Two graft losses occurred, both related to infection. Two additional deaths occurred: one due to lung cancer and the other due to pneumococcal sepsis [47-49].
Samsung Medical Center
Samsung Medical Center group performed eight combined kidney and bone marrow transplants from HLA-mismatched living donors. The first two recipients were conditioned with rituximab, cyclophosphamide, thymic radiation, along with perioperative rATG. The induction protocol was modified in recipients 3 to 5, with reduction of cyclophosphamide, addition of fludarabine, and increased rATG dosage. In recipients 6 to 8, further modifications were made by reducing fludarabine and rATG dosage. After transplantation, maintenance therapy included tacrolimus and steroid in addition to one recipient switching from tacrolimus to sirolimus at month 3. One recipient in protocol 2 achieved long-term IS-free survival for 35 months. All three recipients of protocol 3 achieved IS-free graft survival for 4–41 months with stable kidney function. Both recipients in protocol 1 had engraftment syndrome. Two graft losses occurred in patients 2 and 3. BK virus nephritis was observed in patients 3, 5, and 6 [50].
VCA tolerance studies
Many small animals and large animal studies regarding VCA tolerance have been reported. One study identified that the addition of costimulatory blockade can increase graft survival in mice [51]. Another study utilizing costimulatory blockade in mice identified regulatory T cells as a crucial factor for tolerance induction [52]. A recent VCA tolerance study was reported in a nonhuman primate (NHP) model that attempted a delayed bone marrow transplantation after hand or face VCA transplantation [53]. To date, there exists a significant knowledge gap in how best to avoid immune rejection of human VCAs. It will likely require more scientific advances through research in small and large animal models before tolerance induction should be attempted in human VCA recipients.
LARGE ANIMAL PROTOCOLS
Costimulatory blockade
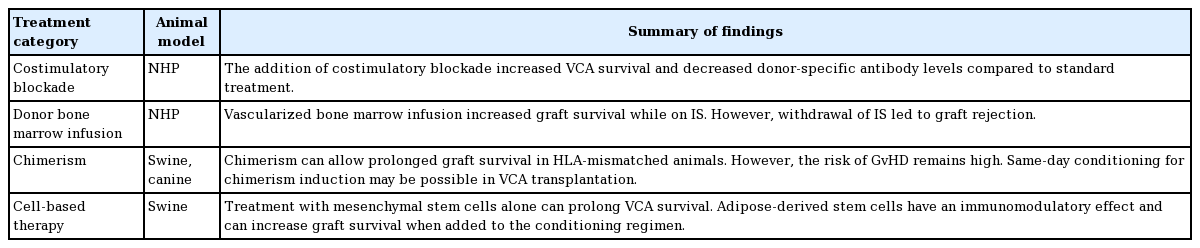
Costimulatory blockade entails interfering with T cell signaling to prevent activation and clonal expansion of T-cells as well as secretion of inflammatory cytokines. T cell-mediated rejection can be inhibited by blocking these signals, leading to prolonged survival of graft tissue. The use of costimulatory blockade in VCA has also been explored by Freitas et al. in a NHP forearm VCA model [54]. They investigated the use of CTLA4-Ig and LFA3-Ig (which blocks the LFA3-CD2 adhesion pathway) in combination or solely with rapamycin compared to tacrolimus alone. The combination of CTLA4-Ig and LFA3-Ig resulted in graft survival but also fatal cytomegalovirus infections in all three animals, and the use of rapamycin led to increased wound complications. However, subjects treated with CTLA4-Ig had prolonged allograft survival and decreased donor-specific antibody levels compared to CNI alone [54]. As with solid organ regimens, it seems that costimulatory blockade will remain an additional medication in the IS regimen in VCA treatment (Table 3).
Infusion of donor bone marrow
Barth et al. [55] speculated that a vascularized bone marrow (VBM) component of a VCA would allow for donor cell engraftment to increase the likelihood of tolerance to the transplanted graft. They compared NHP heterotopic fascial allografts with a VBM component without subsequent tacrolimus and MMF IS. The animals that received the facial allograft with VBM had prolonged survival of the graft while on IS, but rejected their grafts when IS was discontinued. However, alloreactive antibodies were not found in the VBM group, revealing a protective effect of VBM.
In a later study by the same group, Brazio et al. [56] completed a non-pretreated bone marrow infusion at the time of VCA in three experimental animals. They found no protective effects of the bone marrow infusion, and the allografts were rejected as early as postoperative day 28. There were no signs of chimerism in these animals, and alloantibodies were also detected, although to a lesser amount than the group with no donor cell treatments. At this time, 2011 and on, the concept of donor bone marrow presence to promote tolerance to a VCA was continually investigated and refined to the pivotal large animal studies that achieved tolerance across major histocompatibility complex (MHC) barriers.
Stable and transient chimerism
Stable and transient chimerism can be achieved after HCT. HCT is the intravenous infusion of hematopoietic cells containing stem and progenitor cells into the recipient. Once infused, transplanted donor hematopoietic stem cells travel to the recipient’s bone marrow compartment, take up residence, differentiate, and proliferate into all blood lineages. When lineages of donor and recipient cells co-exist, chimerism has been achieved.
Several large animal studies in swine and canines have demonstrated that HCT leading to chimerism is a key factor in VCA tolerance [18]. The first study showing tolerance across MHC barriers using HCT was that by Hettiaratchy et al. [57] in a pig VCA model. The animals were pretreated with a porcine CD3-immunotoxin T-cell depleting agent, split into two groups, and transplanted with cytokine-mobilized peripheral blood mononuclear cells (CM-PBMCs) or BMCs, and treated with a 30-day course of cyclosporine A (CsA). Both groups achieved chimerism, but the CM-PBMC group maintained stable chimerism while the BMC group experienced transient chimerism. The donor musculoskeletal components of three CM-PBMC VCA recipients and two BMC VCA recipients survived long-term, including one fully mismatched animal. Unfortunately, the animals that achieved stable chimerism ultimately developed cutaneous GvHD.
All animals in the study by Hettiaratchy et al. rejected the skin component of the graft, underscoring donor skin immunogenicity as a major obstacle for VCA tolerance [18,57]. Horner et al. [58] investigated the outcomes of a vascularized fasciocutaneous transplant in swine with established stable chimerism, using a conditioning protocol consisting of CD3-immunotoxin, 100 cGy TBI before HCT, and a 45-day course of CsA postHCT that successfully resulted in long-term acceptance of vascularized organ allografts. They achieved complete VCA tolerance for over 300 days in a stably chimeric HCT recipient who had not received IS for 7 weeks, the first published skin tolerance achievement. Leonard et al. [59] further extended this protocol to assess the ability to achieve complete tolerance when the VCA is transplanted at the same time as the HCT. Stable chimerism was achieved, as was tolerance across MHC barriers. However, two animals suffered cutaneous GvHD. The incidence of GvHD was associated with higher levels of donor chimerism.
In a subsequent canine study by Chang et al. [60], BMC- or granulocyte-colony stimulating factor (G-CSF)-mobilized stem cells in peripheral blood were transplanted to recipients preconditioned with 4.5 Gy TBI at a rate of 7 cGy/min. Subjects received VCA and HCT simultaneously, followed by MMF for 28 days and CsA for 35 days. One animal that received BMCs achieved stable chimerism and long-term tolerance of their allograft (52 weeks), while all animals who received G-CSF-mobilized stem cells achieved stable chimerism and long-term tolerance (up to 94 weeks).
The animals in the studies by Hettiaratchy et al. (swine) [57] and Chang et al. (canines) [60] experienced transient chimerism. All animals that received BMC in the study of Hettiaratchy et al. [57] experienced transient chimerism, including one fully mismatched animal with graft survival for 132 days until the graft was purposely removed. The canine that remained tolerant to its graft for 94 weeks in the Chang et al. study lost granulocyte chimerism at 10 weeks and mononuclear cells at 15 weeks [60]. Animals with transient chimerism did not develop GvHD, while animals with stable chimerism did. Swine and canine HCT carry a similar risk of GvHD, and higher donor cells’ levels increase the risk [57,60]. As such, transient chimerism may be a pivotal component to tolerance without the risk of GvHD if it could be harnessed safely and consistently.
Cell-based therapy
Alternative cell infusions have been investigated to avoid the risk of GvHD. Mesenchymal stem cells (MSCs) are multipotent nonhematopoietic progenitor cells residing in the bone marrow. These cells lack immunogenic surface receptors for alloreactive T cells to respond to [61]. Kuo et al. [62] first investigated MSC therapy on outbred swine. They found that with TBI, bone marrow transplant, CsA, and MSC treatment, composite grafts survived for over 200 days in three animals. There were no histological signs of rejection of the skin and muscle at 35 weeks, and none of the animals suffered GvHD. Additionally, they found that with this treatment, regulatory T-cell populations were significantly increased. In their subsequent study, Kuo et al. [63] removed bone marrow transplantation in their tolerance protocol and maintained TBI pretreatment, MSCs, and CsA (n = 3). This protocol led to no signs of rejection in all graft components at the 2- and 6-week time points, and two animals’ grafts survived beyond 100 days. Again, there were no signs of GvHD in any animals. Of note, when swine were treated with solely MSCs in both studies, they experienced increased graft survival compared to no treatment.
Adipose-derived stem cells are harvested from the recipient and require no pre-conditioning, therefore making their use less taxing to the recipient [64]. In adipose-derived stem cell studies, Kou et al. [64] found that outbred swine treated with adiposederived stem cells, tacrolimus, and irradiation (n = 8) had significantly increased graft survival over 196 days. They also found an increase in regulatory T-cells in this group at 6 weeks posttransplant, but these levels normalized by week 15. Cytokine analysis of this group also showed significantly increased transforming growth factor-beta-1 and significantly decreased tumor necrosis factor-gamma. These findings support adipose-derived stem cells’ immunomodulatory effects and their ability to increase regulatory cells and cytokines. Contrary to the MSCs, when adipose-derived stem cells were given as a sole treatment or with CsA after VCA, severe rejection occurred.
DISCUSSION
The constant threat of acute and CR, along with a requirement of intense lifelong IS therapy with a long list of potential sequelae, significantly limits VCAs’ benefit and application [65,66]. Multiple VCA-related deaths have indeed been reported in patients receiving facial allotransplantation [67]. This presents a significant ethical concern regarding the use of VCAs. As it treats non-life-threatening conditions, a comprehensive risk-to-benefit assessment must be done to ensure that the cure is not worse than the condition [68]. Such ethical concerns would be adequately addressed with a successful tolerance induction strategy, eliminating the need for chronic IS treatment.
Clinical tolerance protocols in kidney allograft transplantation provide excellent insights into the future direction of VCA tolerance induction. The major protocols all based their strategy on inducing different levels of donor chimerism in the recipients. The attempts range from the least intrusive of transient chimerism to a more involved mixed chimerism to complete donor chimerism dominance. The Northwestern/Duke group has shown that it may be possible to induce durable chimerism with a relatively low GvHD risk, but the risk remains [47,48]. Transient chimerism would be ideal for avoiding complications if persistent tolerance can be maintained [43]. It may also be that mixed chimerism would strike the perfect balance between persistent tolerance and acceptably low risk of GvHD and other adverse reactions. However, it has yet to be established in HLA-mismatched patients [44-46]. The least intrusive chimerism that would allow for long-term graft survival without IS treatment should be favored. All protocols consistently employ radiation treatment as part of the conditioning regimen along with donor HCT. These treatments are crucial for the induction of tolerance. It is also clear that other adjunct treatments would be necessary to tilt the balance towards a safer and stable tolerance state. It is worth noting that the kidney transplantation tolerance trials were performed with living donors, while VCA donors are likely deceased. This presents an additional barrier to the development of VCA tolerance protocol because there would be minimal time for pre-conditioning. VCA transplantation is also more likely to be HLA-mismatched than kidney transplantation, requiring tolerance induction over more significant immunological barriers.
Large animal models serve as the bridge for new therapies to demonstrate effectiveness before being adopted into human clinical trials [69]. Studies in swine, canine, and NHP continue to provide insight into clinical tolerance protocols. A study done in haploidentical canine models demonstrated the possibility of same-day conditioning for VCA transplantation [60]. The elimination of significant pre-conditioning would open up the potential donor pool for VCA and significantly increase its feasibility and application, as living donor for VCA is unlikely. The study involving belatacept as part of the conditioning regimen for renal allograft transplant in NHP has successfully reported long-term graft survival without IS [70]. VCA transplantation utilizing belatacept has also been shown to increase rejection-free graft survival in NHP [54]. Another promising treatment in large animal studies is T cell depletion through CD3 immunotoxin, which, unlike rATG or alemtuzumab, is capable of depleting T cells within the lymphoid tissues as well as the blood [71,72]. The human equivalent of the porcine CD3-immunotoxin is currently in clinical trials and is being marketed by Angimmune LLC as Resimmune [73]. Recipient T cell depletion using CD3 immunotoxin for VCA transplantation in miniature swine has been shown to allow long-term IS-free graft survival [57,59]. Sphingosine 1-phosphate receptor modulators are another class of medication with therapeutic potential in transplantation patients. One of these is FTY720 (fingolimod), which was reported to reduce cardiac fibrosis in rat studies [71]. However, two phase-3 clinical trials using FTY720 in renal transplantation were performed without meeting the endpoint, and further research was discontinued [74,75]. ASP0028, an S1P1/S1P5 selective agonist, has shown efficacy and safety in an NHP renal transplantation model and could have future therapeutic potential [76].
CONCLUSIONS
The future of the clinical application of VCAs is dependent on shifting the risk-benefit ratio. Reducing or eliminating acute and CR would increase VCA feasibility and applicability by removing the double-edged sword of IS treatment. Animal model studies are crucial for further understanding of the immunological barrier faced with HLA-mismatched VCA transplantation. A successful tolerance induction protocol should contain low toxicity to the recipient, require minimal or same-day pre-conditioning, and utilize short-term IS medications.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: S Colakoglu, CA Huang, DW Mathes. Data curation: JH Yang, AC Johnson. Methodology: CA Huang, DW Mathes. Project administration: S Colakoglu, DW Mathes. Writing - original draft: JH Yang, AC Johnson. Writing - review & editing: JH Yang, S Colakoglu, CA Huang, DW Mathes.