Improvement of Upper Extremity Lymphedema after Delayed Breast Reconstruction with an Extended Latissimus Dorsi Myocutaneous Flap
Article information
Abstract
Lymphedema is a common complication after mastectomy in breast cancer patients. Many treatment options are available, but no treatment results in a complete cure. We report a case of lymphedema that occurred after modified radical mastectomy in a breast cancer patient who showed objective improvement after delayed breast reconstruction with an latissimus dorsi myocutaneous flap. A 41-year-old female patient with left breast cancer had undergone modified radical mastectomy with axillary lymph node dissection and postoperative radiotherapy 12 years previously. Four years after surgery, lymphedema developed and increased in aggravation despite conservative treatment. Eight years after the first operation, the patient underwent delayed breast reconstruction using the extended latissimus dorsi myocutaneous flap method. After reconstruction, the patient's lymphedema symptoms showed dramatic improvement by subjective measures including tissue softness and feeling of lightness, and by objective measures of about 7 mL per a week, resulting in near normal ranges of volume. At a postoperative follow-up after 3 years, no recurrence was observed. Delayed breast reconstruction with extended latissimus dorsi myocutaneous flaps may be helpful to patients with lymphedema after mastectomy. This may be a good option for patients who are worried about the possibility of the occurrence or aggravation of secondary lymphedema.
INTRODUCTION
Lymphedema is abnormal, regional accumulation of protein-rich fluid in the interstitial space that can result in edema formation and chronic inflammation [1]. Lymphedema mainly occurs as a result of the malfunction or nonfunction of lymphatic circulation and is commonly classified as primary or secondary. Lymphedema after mastectomy in breast cancer patients is a common type of secondary lymphedema, with a reported prevalence of 10% to 20% of all mastectomy patients [2,3]. It often occurs after postoperative axillary radiotherapy and procedures performed to the axillary region during the operation, including axillary lymph node dissection and even sentinel lymph node biopsy.
Treatment options for lymphedema include conservative management or surgical treatment using a supermicrosurgical technique; however no complete cure for lymphedema is known.
Reconstruction using an extended latissimus dorsi mycocutaneous flap is widely used, especially in Asian women who have small breast volumes and do not have excessive abdominal fatty tissue. However, harvesting this flap requires identifying thoracodorsal vessels for a pedicle, and dissecting the axillary region that was dissected during the previous operation. Redissecting the axillary area may be traumatic to the axillary lymphatic circulation including the lymph node, and may lead to occurrence of lymphedema or aggravation of preexisting lymphedema.
A few studies of patients with lymphedema report symptom improvement after delayed reconstruction with autologous tissue [1,4]. However, objective assessments have not been included in most reports and few reports are available on the relationship between lymphedema improvement and delayed breast reconstruction with an extended latissimus dorsi myocutaneous flap.
We report on a patient with preexisting lymphedema after mastectomy whose symptoms improved after delayed breast reconstruction with an extended latissimus dorsi myocutaneous flap.
CASE
A 41-year-old female patient visited the Department of Plastic Surgery for delayed breast reconstruction. She had received a modified radical mastectomy and postoperative radiotherapy 12 years previously for left breast cancer. Four years after surgery, lymphedema developed in her left hand, which was also identified by objective measurement of volumetry (Perometer type 350s, Nambuk Surgical, Seoul, Korea) (Fig. 1). A perometer is an electromechanical device used to calculate the limb volume with the data gathered electronically from the limb inserted into a vertically- or horizontally-oriented frame that emits infra-red light beams at right angles to each other. Despite conservative treatment, including manual lymph drainage and compressive garments, the symptoms worsened and the patient's entire left arm was affected. She requested delayed breast reconstruction with autologous tissue. Because she did not have a large volume of breast material on the contralateral side and did not have excessive abdominal tissue, the patient underwent breast reconstruction with an extended latissimus dorsi myocutaneous flap. The flap was harvested with a 25×10 cm skin paddle and 30×30 cm fat extension flap. After the thoracodorsal vessel was identified and skeletonized to acquire a sufficient arc of flap rotation, and the humeral insertion of the latissimus dorsi muscle was resected, the flap was inset to the left chest wall. The patient was discharged at postoperative 10 days with no complications.
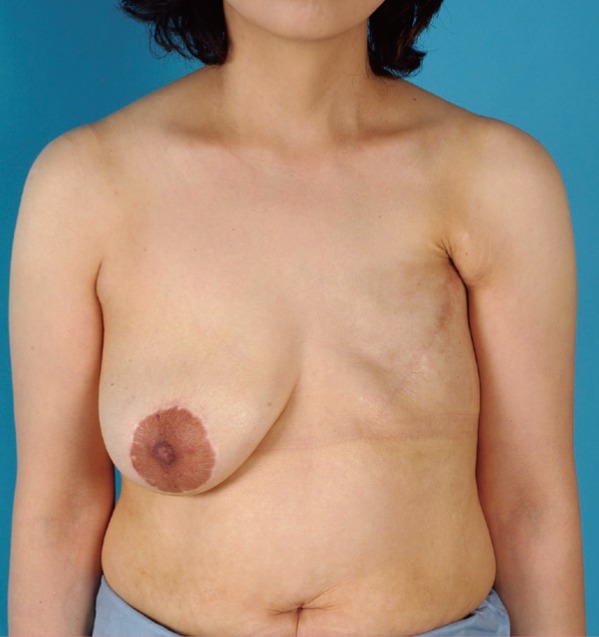
Preoperative status
A 41-year-old female patient had received a modified radical mastectomy and postoperative radiotherapy 12 years previously for left breast cancer. Four years after surgery, postmastectomy lymphedema developed in her left hand and forearm.
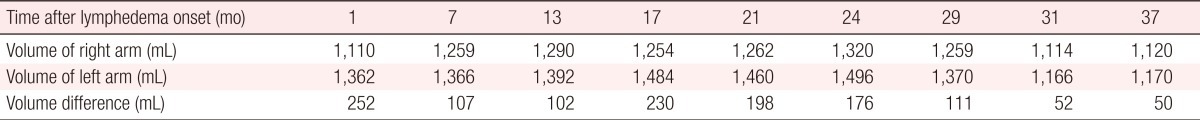
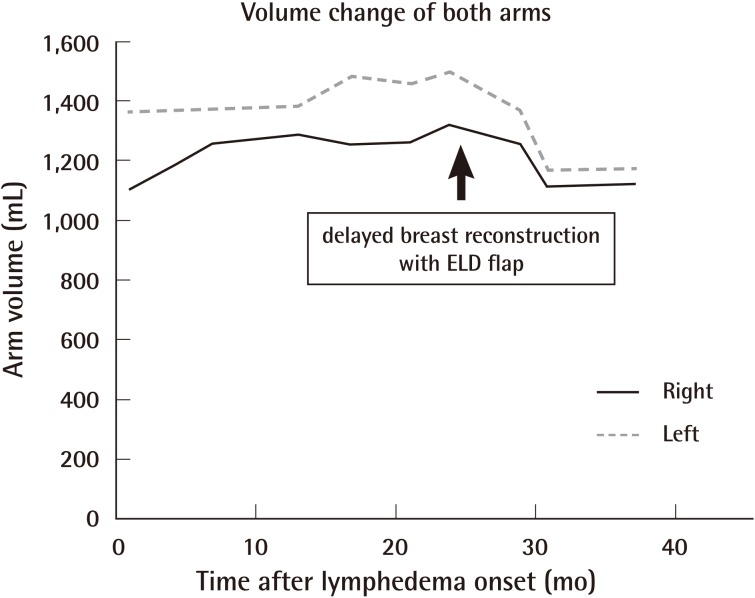
At 2 months after the delayed breast reconstruction, the patient's lymphedema symptoms began to improve by subjective measures (Fig. 2), and after 4 months, the degree of improvement was identified by the objective assessment of volumetry (Table 1). The volume of the affected arm decreased continuously at a rate of about 7 mL per week, which was faster than that of the pre-reconstructive state (Fig. 3). At one year after the operation, the volumes of the two arms were nearly the same and no recurrence was observed at follow-up 3 years after the operation.
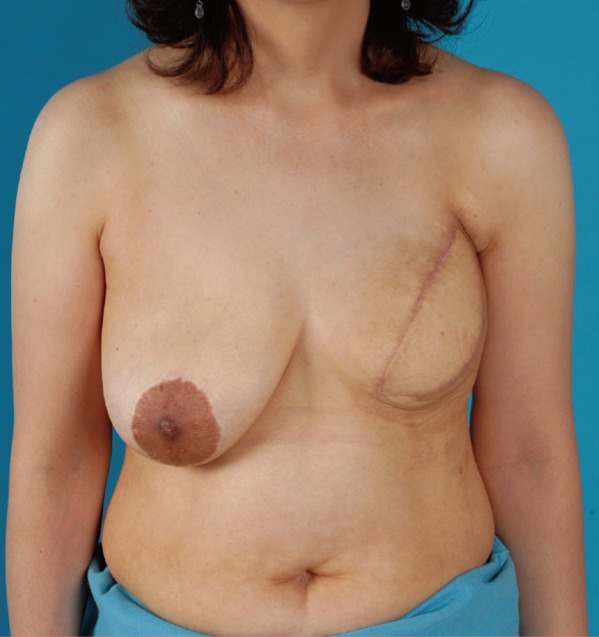
Postoperative 2 months status
The patient's lymphedema symptoms began to improve by subjective measures at 2 months after the breast reconstruction, and after 4 months, the degree of improvement was observed by the objective assessment.
DISCUSSION
Several risk factors induce lymphedema after mastectomy. First, postoperative radiotherapy at the axillary region can result in lymphedema. Radiation without surgery can increase the risk of lymphedema, and the prevalence of lymphedema is higher in patients who undergo mastectomy followed by radiotherapy [5,6]. The type of mastectomy surgery can affect the occurrence of lymphedema. Generally, radical mastectomy induces lymphedema more often than limited surgery such as lumpectomy. The degree of invasiveness of axillary lymph node surgery can also affect the rate of lymphedema occurrence. The higher the level of axillary dissection, and the more the axillary lymph node is resected, the higher the rate of lymphedema [6,7]. Even sentinel lymph node biopsy in which only a few suspicious lymph nodes are resected can induce lymphedema. The procedure may affect lymphatic circulation, leading to lymphedema occurrence. In addition, obesity with a body mass index exceeding 25 and age over 60 can be risk factors for lymphedema [6].
The most common lymphedema treatment is nonoperative, conservative treatment including manual lymph drainage or intermittent compression therapy. Decongestive lymphatic therapy is the most popular treatment and includes manual centripetal lymphatic massage, compressive bandaging or garments, and exercises [6]. Medications such as benzopyrone 5, 6-benzo-a-pyrone or coumarin, or lymphocyte intraarterial injection, can enable lymphatic flow and normal proteolysis by macrophages, leading to the removal of excessive protein trapped in the interstitial fluid with eventual symptom improvement [6]. However, these treatments may have a limited effect in some patients and surgery may be attempted in patients intractable to conservative management. Surgical treatment can include excisional methods or bridging techniques [4,6]. Excisional surgery removes all excessive and abnormal tissue to decrease the volume of the affected extremity. However, sacrifice of normal tissue is unavoidable and can result in aesthetic and functional disruption. Bridging techniques have developed with supermicrosurgery techniques, and include lymphaticovenular anastomosis, which brings together a lymphatic channel with a subdermal venule, lymphatic vessel transfer, and lymph node transfer [6]. However, this treatment does not cure symptoms completely, and even if the surgery is successful, it must be combined with conservative treatment. Other bridging operations include inserting healthy tissue into the region where the lymphatic flow was damaged to induce regeneration of lymphatic channels.
A few reports indicate that transfer of a free flap or pedicled flap can assist local lymphatic regeneration. Slavin et al. [8] reported 10 patients with free flap reconstruction of extremity wounds who received radiocolloid lymphoscintigraphy between 8 and 44 days after surgery, and demonstrated that lymphatic drainage was reestablished after flap transfer. In addition, lymphedema after mastectomy in breast cancer patients is reported to improve after delayed breast reconstruction. Chang and Kim [1] reported that when patients with lymphedema followed by breast cancer operation received delayed breast reconstruction, lymphedema was not aggravated and symptoms were improved in 23% of patients, regardless of flap type, recipient vessel, or whether the flap was pedicled or free. However the study was limited by lack of objective and quantitative assessments about the degree of lymphedema. Abbas Khan et al. [4] reported that lymphedema improved in breast cancer patients who developed postmastectomy lymphedema after delayed breast reconstruction with an ipsilateral latissimus dorsi pedicled flap and silicone implant, as determined by the objective method of volumetry. However, foreign bodies such as silicone implants were used in combination with the muscle flap, in contrast to the case we report, in which only autologous tissue was used for breast reconstruction.
In this case, the lymphedema was confirmed to be dramatically improved after delayed breast reconstruction with an extended latissimus dorsi myocutaneous flap. The rate of decline was 7 mL per a week, which was remarkably faster than that in the preoperative state. No aggravation or recurrence of symptoms was observed, even in long-term follow-up. We assume that healthy tissue like muscle tissue with a fat extension flap was interposed into the region where the lymphatic flow had been damaged in the previous surgery, and induced the regeneration of lymphatic channels.
This hypothesis cannot be supported by only a single case. However, it can show that the extended latissimus dorsi flap should not be excluded from the options for delayed breast reconstruction simply because it may induce the occurrence or aggravation of lymphedema because the procedure requires additional dissection at the axillary region. Rather, this flap has other advantages over previous techniques, such that insertion of healthy muscle tissue in the axillary region may help lymphatic regeneration and reduce lymphedema symptoms. Thus, the extended latissimus dorsi myocutaneous flap is a good option, especially in patients who are worried about the possibility of occurrence or aggravation of secondary lymphedema.
Notes
No potential conflict of interest relevant to this article was reported.