Neuroendocrine Differentiation of Primary Mucinous Carcinoma of the Cheek Skin
Article information
Primary mucinous carcinoma of the skin (MCS) is a rare sweat gland tumor, with approximately 175 cases reported in the literature since the first case was described by Lennox et al. [1] in 1952. Although MCS is usually slow growing, it often shows locally aggressive behavior and a high rate of local recurrence following simple excision. The clinical appearance and differential diagnosis of MCS vary, but histopathologically MCS is similar to metastatic carcinomas, specifically of the breast and colon. The recognition of MCS is essential for preventing an erroneous diagnosis of metastatic carcinoma. In Korea, to our knowledge, only 1 of 9 cases that have been reported was diagnosed as neuroendocrine differentiation [2]. Kim et al. [2] reported a case of primary mucinous carcinoma with neuroendocrine differentiation, focusing on its clinical and histologic features. In the present study, we report a case of MCS with neuroendocrine differentiation on the cheek that was treated with wide excision and a rotation advancement cheek flap, which showed favorable results. This article is focused on the distinctive pathological findings, the surgical approach, and the postoperative evaluation process of neuroendocrine differentiation of MCS.
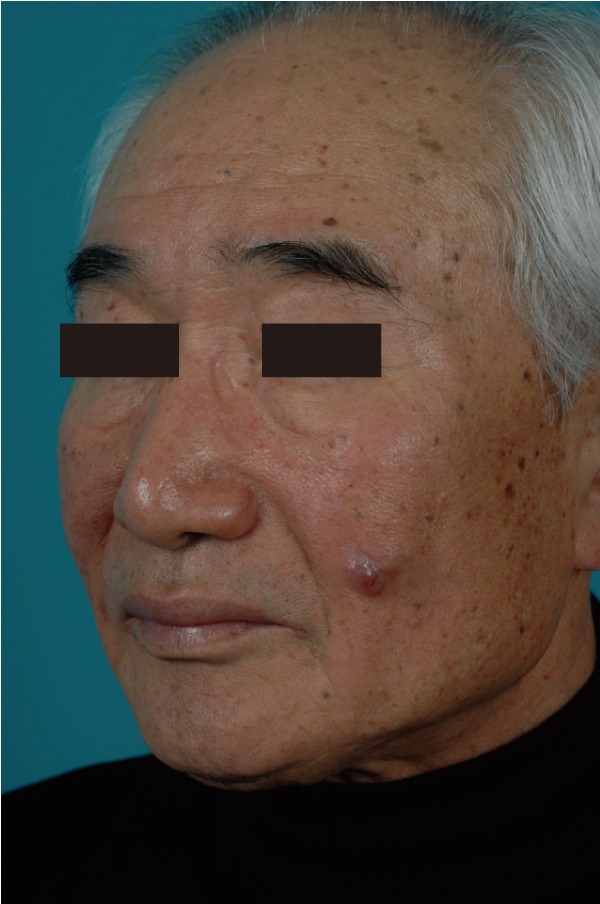
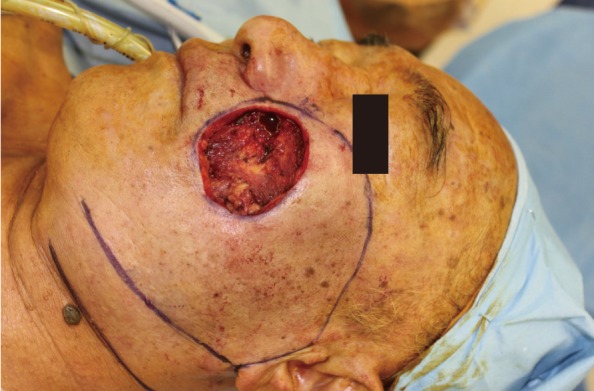
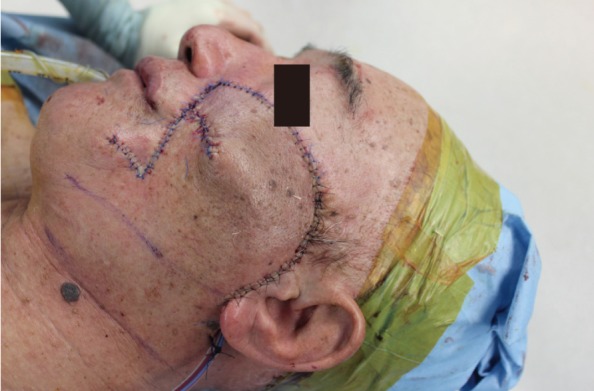
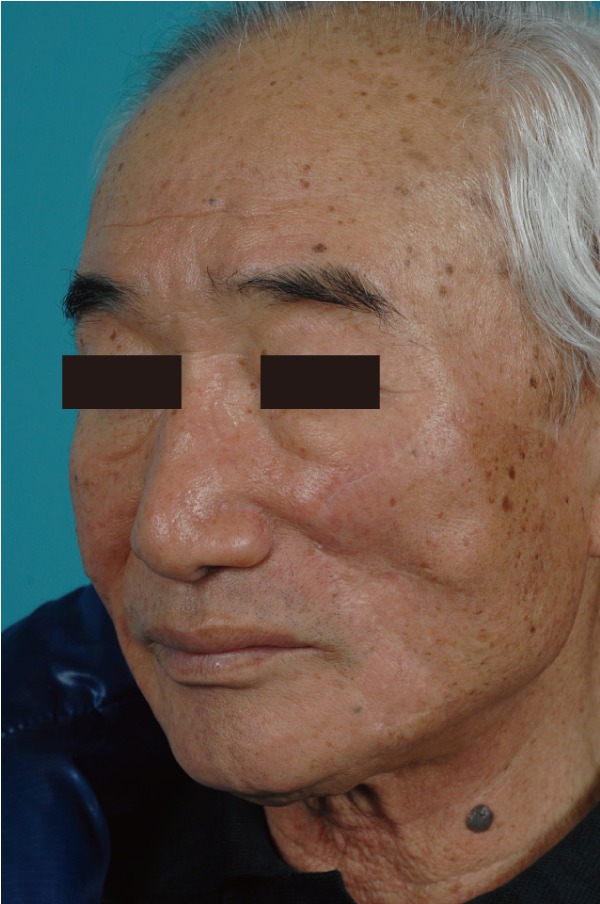
A 72-year-old man presented with a 3-year history of an asymptomatic 15×20 mm, well-demarcated, erythematous nodule growing on his left cheek (Fig. 1). He had first noticed this lesion 3 years prior and became concerned as it slowly enlarged. An incisional biopsy demonstrated mucinous eccrine carcinoma with positive margins. Under general anesthesia, the tumor was excised with 2 cm margins, according to oncological principles, as a full-thickness specimen. The regional lymph nodes were not involved. The surgical defect measured 5.5×6.0 cm (Fig. 2). Although he was an elderly patient, his skin laxity was limited. Therefore, extensive undermining was performed in the surrounding facial region in the subcutaneous plane and stopped at the lower border of the mandible; there was no undermining in the cervical region. The rotation flap was progressively elevated until there was adequate flap mobilization to cover the defect area and tension-free closure was possible (Fig. 3). The histologic examination revealed aggregates of glandular epithelioid cells floating in mucinous pools that were defined by thin, fibrous septa. The pools of mucin stained positive for Alcian blue at pH 2.5 and periodic acid-Schiff. Immunohistochemical staining demonstrated positive reactions to synaptophysin (Fig. 4), estrogen receptors (ER), progesterone receptors (PR), and cytokeratin 7, and a negative reaction to cytokeratin 20, which met the description of markers positive for neuroendocrine differentiation. A full metastatic workup was performed, including computed tomography (CT) and positron emission tomography (PET) scans, without evidence of another primary type of cancer. Two years after surgery, the patient remains in good health without recurrence, showing good aesthetic results (Fig. 5).
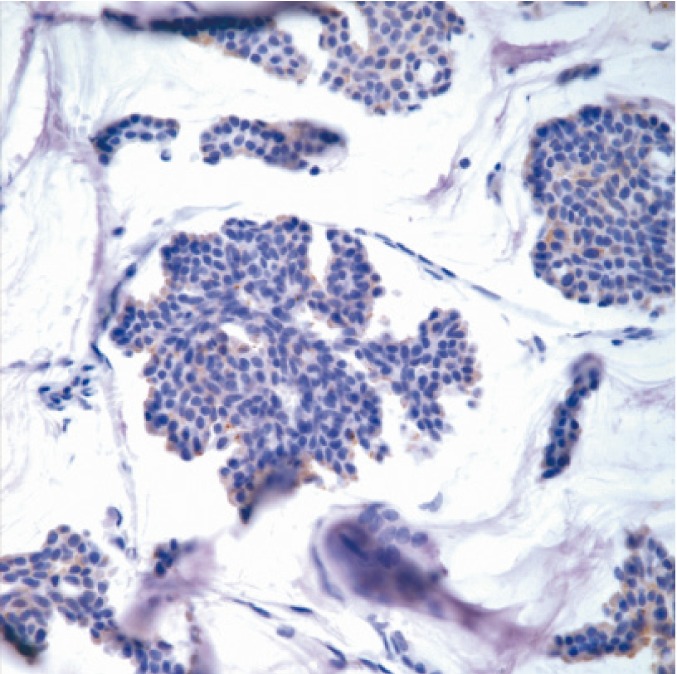
Immunohistochemical findings of the neuroendocrine marker: synaptophysin ×400; positive staining was observed.
Histologically, MCS may appear similar to other sweat gland tumors and metastatic carcinomas. Immunohistochemical staining of MCS cells with Alcian blue, which shows positive at a pH of 2.4, but negative at a pH of 4.0, can differentiate MCS from other sweat gland tumors [3]. Gastrointestinal carcinomas contain sulfomucin, while sialomucin is present in MCS [3]. Cytokeratin 20 is common in gastrointestinal neoplasms, but absent in MCS [3]. Cytokeratin 7 and p63 can be found in MCS, but are rarely found in metastatic tumors [3]. Positive immunohistochemical staining for synaptophysin, cytokeratin 7, ER, and PR with a negative reaction to cytokeratin 20, indicates neuroendocrine differentiation [3]. Since 1980, neuroendocrine differentiation of mucinous carcinoma of the breast has been reported according to a type A (without neuroendocrine differentiation), type B (with neuroendocrine differentiation), and type AB (intermediate form) classification [4]. Two previous studies have shown that good prognosis was found in mucinous carcinoma of the breast showing neuroendocrine differentiation [4]. The exact component ratio of neuroendocrine differentiation among mucinous cutaneous carcinoma has not been reported yet [2]. A full oncologic evaluation to search for other primary cancer sites is required for all patients with sweat gland tumors to distinguish MCS from metastatic tumors.
MCS is unresponsive to chemotherapy and is radiation-resistant. Given the high rate of local recurrence and the low rate of distant metastases, the gold standard treatment is excision of the tumor. Although local excision with narrow 2 mm margins is sometimes sufficient for superficial tumors, local recurrence has been reported following excision with a 1 cm margin in at least one case. There has been one case report with no evidence of recurrence or metastasis for 10 months postoperatively following resection with 1.5 cm to 2.0 cm margins [5].
For small cheek skin defects (less than 4 cm), the cheek rotation flap described by Mustarde generally allows for easy coverage without complications. When there is no skin laxity, a more extensive undermining in the facial region is necessary to cover the defect [5]. Cervicofacial flaps are helpful for larger cheek skin defects with a wider flap elevation and undermining. However, the resultant closure often has tension when occurring on the cheek, requiring larger incisions and undermining, which can result in compromised vascularity and vulnerability to ischemic necrosis on the distal end of the flap. For the 6 cm cheek defect in our case, the rotation advancement cheek flap with subcutaneous undermining only to the mandible border while preserving the cervical region allowed for good coverage without complications. The flap color and texture was well matched with the surroundings without flap loss.
The slow growing and clinically asymptomatic features of MCS can delay the diagnosis, and to rule out other sources of metastatic mucin-secreting carcinomas to the skin, an imaging scan (CT, magnetic resonance imaging [MRI] or PET) is always required. Due to the locally aggressive behavior and high rate of local recurrence of MCS, surgical excision with a wide safety margin is required, and an annual follow-up with detailed systemic examination is recommended. For MCS with local recurrence and lymph node metastasis, whole-body CT and MRI is necessary.
In conclusion, MCS is a rare sweat gland malignancy, and only one case was previously reported in Korea with neuroendocrine differentiation. To rule out other sources of metastatic mucin-secreting carcinomas to the skin mimicking MCS, a full metastatic workup is necessary. Due to the locally aggressive behavior and high rate of local recurrence of MCS, surgical excision with a wide safety margin is required, and an annual follow-up with detailed systemic examination is recommended.
Notes
No potential conflict of interest relevant to this article was reported.