Chest Wall Fibromatosis in the Axilla
Article information
Fibromatosis is a rare tumor that is caused by the proliferation of well-differentiated fibroblasts. Two-thirds of all fibromatosis is abdominal. After the abdomen, the shoulder and pelvis are the parts of the human body where fibromatosis occurs most frequently [1]. It invades adjacent tissues and has a recurrence rate of 10% to 90% after excision, even though it is histologically a non-metastatic benign tumor. While fibromatosis in the shoulder, pelvis, mesenterium, head, and neck have been reported, fibromatosis in the axilla is very rare. We report a case of chest wall fibromatosis with left arm involvement that was successfully treated by function-preserving surgery.
A 39-year-old female had experienced paresthesia including numbness for 2 years and by chance was found to have a mass at the left axilla during a breast ultrasonography. As the patient had no accompanying symptoms with the left arm numbness, conservative treatment was carried out with monitoring of progress. During the conservative treatment, it was noted that the frequency and intensity of numbness gradually increased and a limited range of motion of the left arm was noted.
Physical examination of the left axilla was unremarkable. However, upon careful palpation, a 2×5 cm hard, fixed, oval-shaped painless mass was detected. Erythema or a heating sensation and tenderness were not evident surrounding the mass (Fig. 1). Laboratory and radiologic findings showed no abnormality. On T1 weighted magnetic resonance imaging, a 3×7 cm benign tumor with low signal intensity tightly connected to the chest wall and positioned in front of the subscapularis muscle of the left axilla was observed. Invasion of the chest wall and ribs were not noted and the major neurovascular bundle was not invaded (Fig. 2).
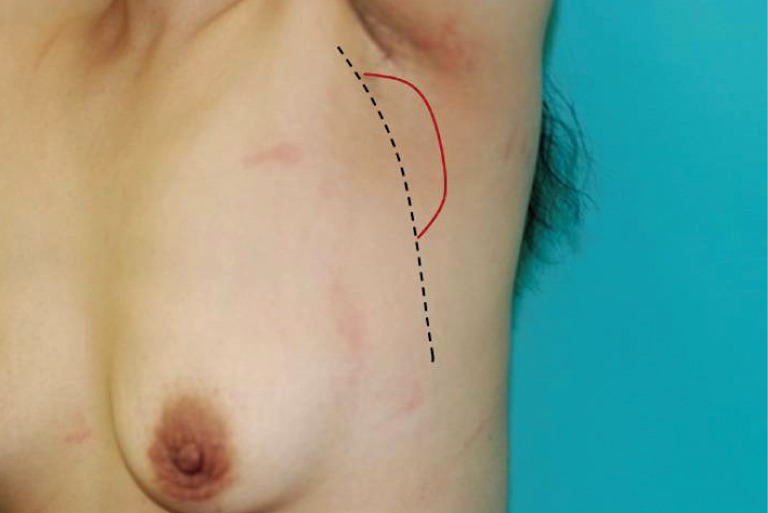
A 5 cm mass that was not visibly detectable was revealed by palpation. The frequency and intensity of numbness of the left arm gradually increased and newly accompanied by a limited range of motion. Dashed line, pleura wall; red line, mass.
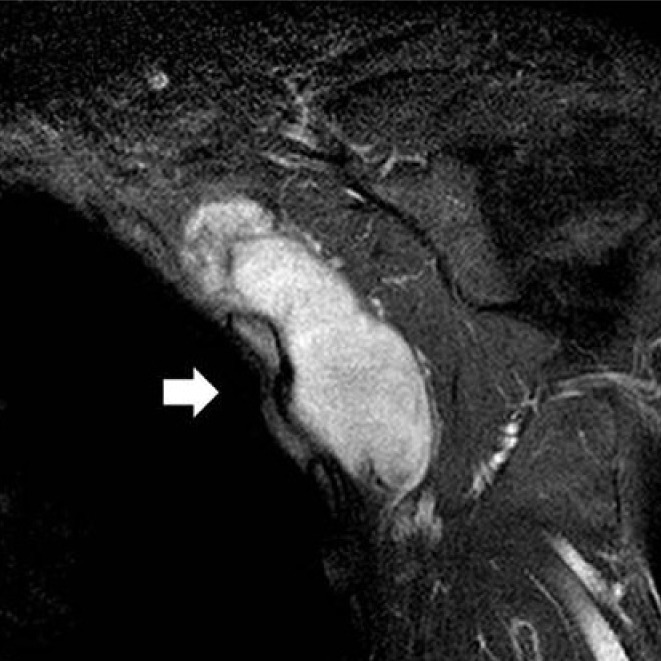
Preoperative magnetic resonance image. A 3×7 cm well-circumscribed mass (white arrow) was seen without invasion of the chest wall or adjacement ribs (coronal T1 weighted Gadolinium image).
Under general anesthesia, for a sufficient operative view, an S-shaped skin incision was made and displaced medially. Following the anterior displacement of the pectoralis major and minor muscles, the mass, except the vital structure area, was exposed and excised by the Enneking staging method [2]. The intercostal muscle and perichondral tissue were also removed together, preserving the pleura and brachial plexus. This mass was firm without crumbling and was removed with splitting to attain a safe margin and wide operative view. The 3×7 cm mass showed a relatively rough surface that adhered to the surrounding organs tightly (Fig. 3). The mass was confirmed to be benign by intraoperative frozen sections. It was pathologically regarded as a result of the proliferation of uniform spindle-shaped cells (Fig. 4). The immunohistochemistry for smooth muscle actin and S-100 protein was negative. The mass was diagnosed as fibromatosis. Paresthesia including left arm numbness disappeared immediately after the operation; no recurrence has been evident during the ensuing 1 year (Fig. 5). As this tumor frequently occurs in patients with a history of familial adenomatous polyposis, endoscopic examination of the large intestine was performed. No abnormality was evident.
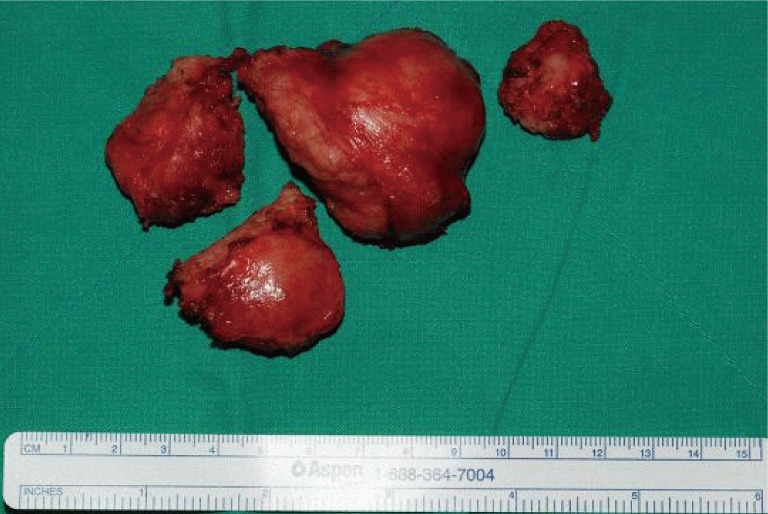
The fragmented masses are firm, nodular, and encapsulated by membranous tissue and have a fibrous adhesive margin.
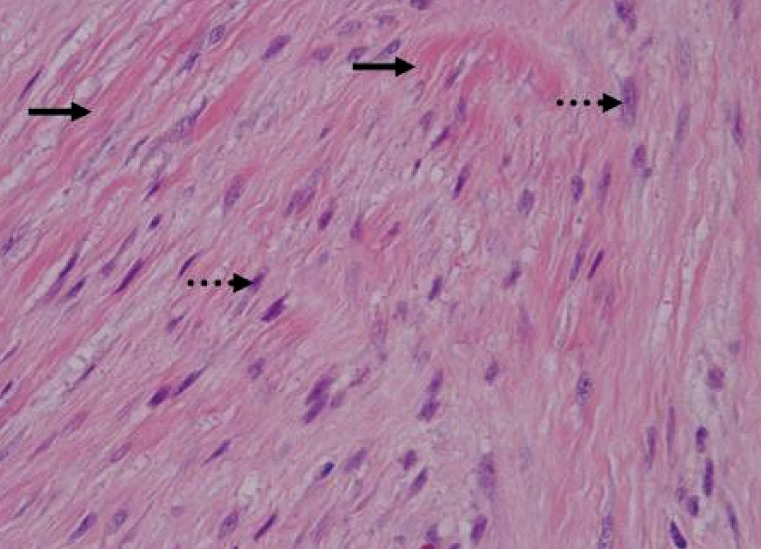
Microscopically, the tumor was composed of abundant collagen (black arrows) surrounding poorly circumscribed bundles of spindle cells (dashed arrows). The dense bundles of eosinophilic spindle cells contain regular nuclei and pale cytoplasm with neither mitoses nor giant cells (H&E, ×400).
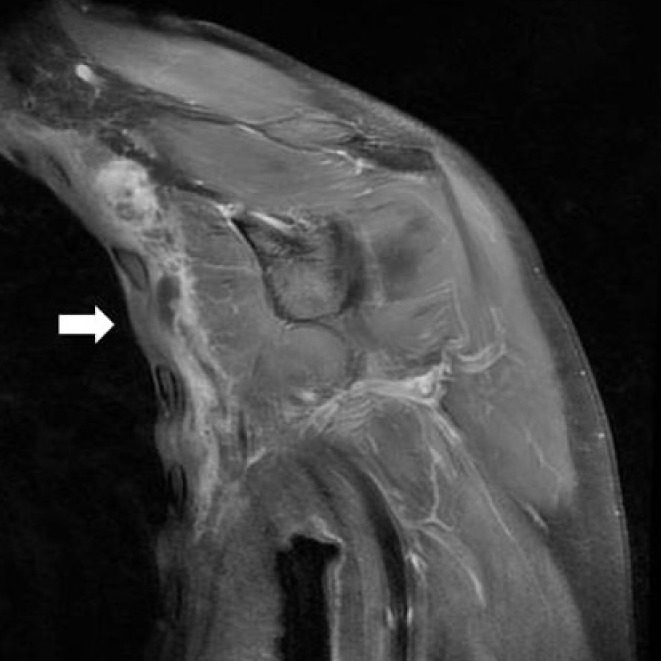
Postoperative magnetic resonance image. No mass remnant (white arrow) was found and minimal fluid collection was noted (coronal T1 weighted gadolinium image).
Fibromatosis is a rare benign disease that comprises 0.03% of all solid masses and 3.6% of fibrous tissue tumors. It occurs more frequently in males than in females but has a relatively high incidence in females of reproductive age, as in the present case, so a relationship between the cause of incidence or tumor growth with dyscrinism and pregnancy seems evident. External injury is also regarded as a major cause. A genetic defect may be a cause of fibromatosis, since the condition occurs frequently in patients with genetic defects in growth regulation of connective tissues, such as familial adenomatous polyposis.
Fibromatosis is a painless hard tumor. As it gradually grows in size, it may impact the surrounding tissue and symptoms may occur, as in this case. In this case, paresthesia, including left arm numbness and a limited range of motion of left arm, resulted, while the tumor grew to have a mass effect in the brachial plexus. The mass effect was confirmed by the postoperative disappearance of the symptoms in the left arm. The differential diagnosis typically includes vascular and soft tissue tumors such as fibrosarcomas or neurofibromas. Computed tomography scanning and magnetic resonance imaging are used for the diagnosis and follow-up of fibromatosis.
Pathologically, fibromatosis has its own growth pattern and has more differentiated and less mitotic cells than fibrosarcoma does, and has relatively less mitosis. Well-differentiated fibroblasts proliferate, grow, and infiltrate adjacent tissues, and abundant collagen is found. It has no cytological malignanacy and no remote metastasis occurs, but it tends toward frequent recurrence.
Wide excision is the most effective method. However, typically a tumor has a large size and an unclear margin, so it is difficult to treat with wide excision. Treatment is very difficult when the tumor is adjacent to the chest wall and too close to the neurovascular bundle, as occurred in the present case. Merchant et al. [3] supported the use of function-preserving surgery, in which the main structure such as main nerve trunk has to be preserved through it is included in the resection margin. They reported that no statistically significant difference in recurrence rate was found in comparison with the group receiving wide excision. Therefore, the authors also excised using function-preserving surgery with minimal damage to the surrounding tissue and, particularly due to severe limitation of operation space, splitting was used for complete excision. This was possible because the mass was diagnosed as a benign tumor in the frozen biopsy. Recurrence or other complications have not been observed during the 1-year postoperative observation period, and conservative therapy is being maintained.
Anticancer chemical drug treatment is used to effectively reduce the size of a mass, rather than to cure, when excision is not recommended or before an excision operation [4]. Anthracycline is the most effective medicine and tyrosine kinase inhibitor the least effective [5]. The effectiveness of radiological treatment is contentious. It may be used to prevent recurrence or when excision is impossible. The range of 50 to 60 Gy is usually effective with side effects such as joint stiffness and neuropathy. Other medical treatment including non-steroidal anti-inflammatory drugs require additional research, as different reports represent different evaluation results.
Notes
This article was noted as a poster at the 1st Research & Reconstructive Forum on May 12-13, 2011 in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.