Primary Intraosseous Hemangioma in the Frontal Bone
Article information
Intraosseous hemangioma is a rare bone tumor accounting for 0.7% to 1.0% of all bone tumors. It can occur at all ages but is most common in the fourth and fifth decades of life and has a female preponderance (3:1) [1]. Intraosseous hemangiomas are usually found in the vertebral column and rarely seen in the calvarium. Among the calvarial bones, the parietal bone is the most commonly involved, followed by the frontal bone and less frequently by the occipital and temporal bones [2]. The pathogenesis is still unknown but a history of trauma seems to be related in some cases reported in the literature [3].
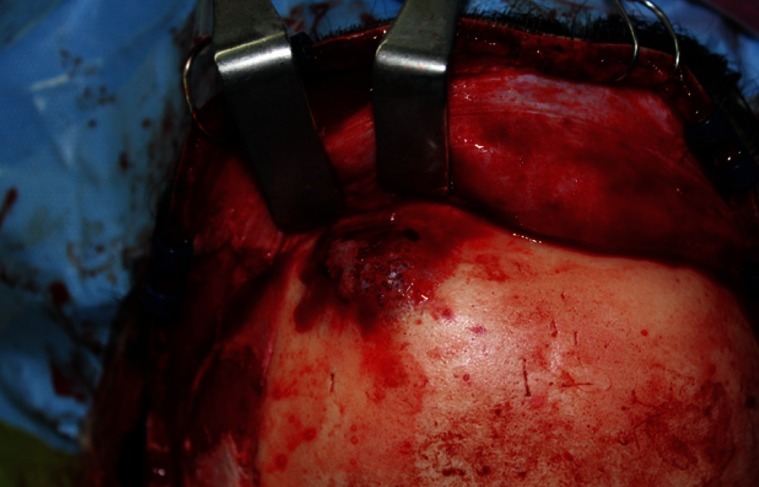
A 39-year-old woman presented with a swelling on the left forehead, which had been slowly enlarging for one year. She had a history of minor trauma on her forehead one-and-a-half years earlier and she seemed to believe that her swelling was related to her previous head trauma. Other medical history and a review of systems were insignificant. The mass was bony hard, tender, and measured approximately 3×3 cm. A plain skull X-ray showed a radiolucent lesion on the frontal bone and a bone window computed tomography (CT) scan demonstrated a 3×3.5×1.5 cm intradiploic osteolytic mass adjacent to the left frontal sinus wall and the orbital roof (Fig. 1). The mass was interpreted as a fibrous dysplasia by a radiologist. Under general endotracheal anesthesia, a bicoronal incision was made, and the mass was exposed. The mass had a hemorrhagic feature, which contained a cluster of small vessels replacing the bony structure (Fig. 2). The mass expanded externally and intracranially, and the inner surface of the mass had loosely adhered to the dura mater. The mass, along with 0.5 cm of normal bone margin, was removed en bloc by craniotome. The frontal sinus wall and orbital roof were preserved intact after the removal of the mass. The bony defect was reconstructed with two pieces of split calvarial bone harvested from the right parietal bone (Fig. 3). A histologic examination of the specimen revealed an intraosseous cavernous hemangioma characterized by extended, thin-walled vessels and sinuses lined with a single layer of endothelial cells (Fig. 4). The postoperative course was uneventful and the contour of the forehead was restored well. There was no recurrence of the tumor visible on a CT scan 9 months after the operation (Fig. 5).
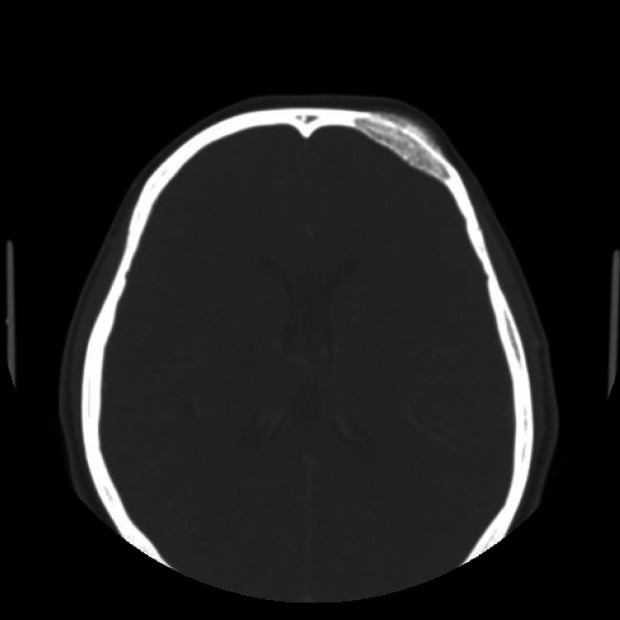
Preoperative computed tomography (CT) scan. A bone window CT scan demonstrated a 3×3.5×1.5 cm intradiploic osteolytic mass adjacent to the left frontal sinus wall and the orbital roof.
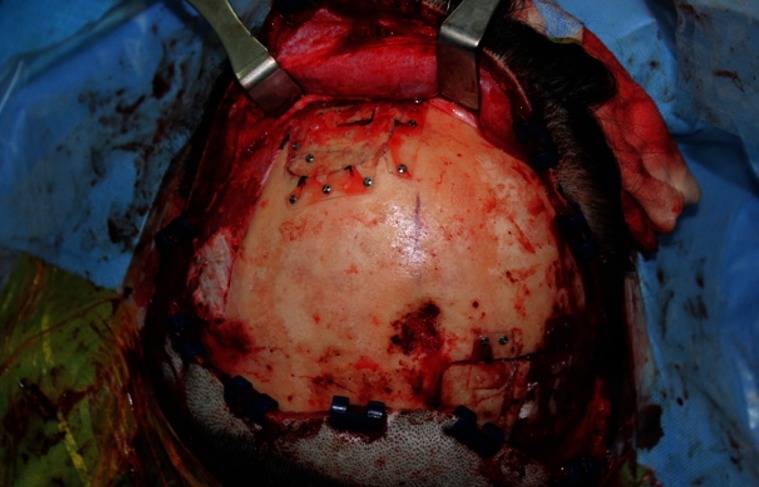
The bony defect was reconstructed with a split calvarial bone harvested from the right parietal bone.
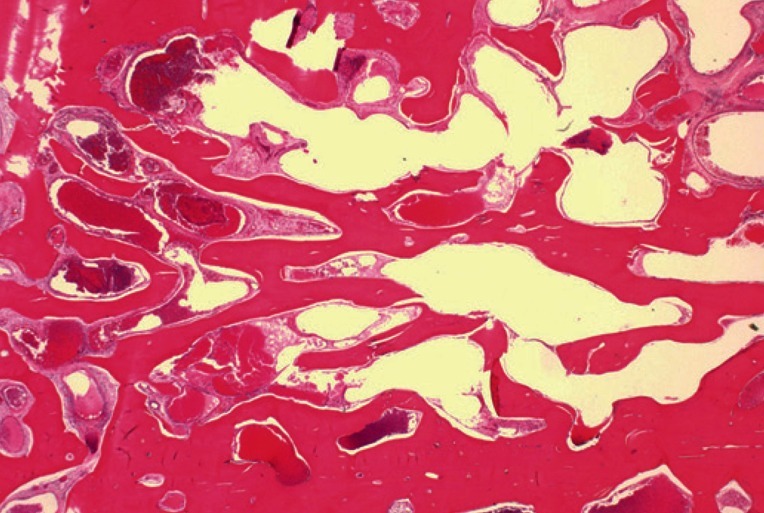
Microscopic findings with H&E stain: cavernous hemangioma showing extended, thin-walled vessels, and sinuses lined by a single layer of endothelial cells (×12).
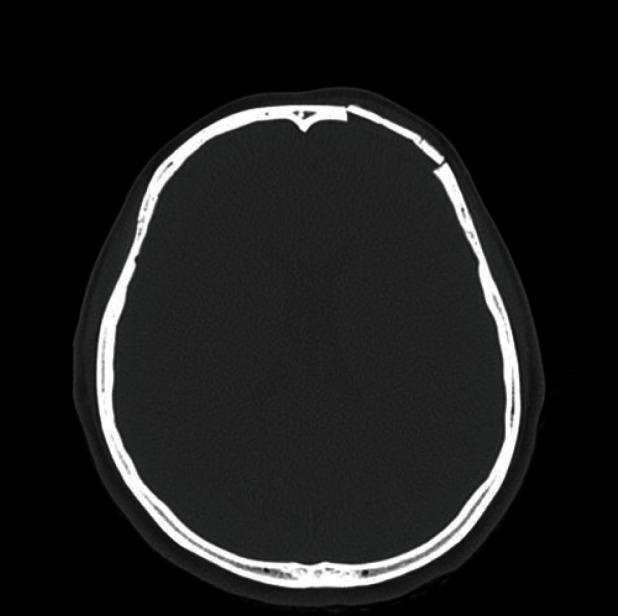
Postoperative computed tomography scan after 9 months of follow-up: there was no remnant mass or recurrence at the cranioplasty site.
The pathogenesis of primary intraosseous hemangioma remains unknown. The cause is considered to be congenital or traumatic, but this has not yet been proven. Trauma is considered to be the most important etiology of this tumor. The symptom is a slowly growing palpable hard mass with mild tenderness. A neurologic deficit is unusual because these tumors tend to expand externally rather than internally although intracranial expansion has been reported [4]. In our case, since the tumor mass had expanded bidirectionally and the inner surface of the mass had loosely adhered to the dura mater, careful dissection between the tumor and dura was needed after the craniotomy. Depending on the sites involved, various symptoms can occur. Loss of vision and proptosis have appeared when the bone lesion involves the orbit. In mandibular or maxillary bone involvement, excessive bleeding can occur as a major complication at the time of surgery and after tooth extraction. Facial nerve paralysis or hearing loss can develop with temporal bone involvement of intraosseous hemangioma. Radiologic evaluation includes plain skull X-rays, CT scan, and magnetic resonance imaging (MRI). The radiologic characteristics of intraosseous hemangioma are an osteolytic lesion replacing normal bone tissue. A CT scan is the most accurate tool for determining the intraosseous extent and soft tissue involvement. However, it is not easy to make a definite diagnosis of intraosseous hemangioma by radiologic evaluation because many conditions involving bone tissue can appear as an osteolytic defect with a "soap bubble," "honeycomb," or "sunburst" appearance. These radiologic phenomena result from the osteolytic activity of the tumor mass and secondary reactive osteoblastic remodeling with trabecular bone. Therefore, the differential diagnoses for intraosseous hemangioma include fibrous dysplasia, osteoma, Langerhans cell histiocytosis, and multiple myeloma. In particular, fibrous dysplasia can be distinguished from intraosseous hemangioma by CT scan. Fibrous dysplasia exhibits a "ground glass appearance;" on the other hand, intraosseous hemangioma demonstrates a "honeycomb" appearance, which is the scattering of radiodense spots within the radiolucent hollow. However, when intraosseous hemangioma is not suspected, it could be often misdiagnosed as fibrous dysplasia.
MRI can also be helpful for evaluating intraosseous hemangioma. The signal intensity is variable and depends on the amount of fat tissue in the bone marrow and blood flow in the channel of dilated blood vessels. On T1-weighted imaging, small lesions show increased intensity whereas larger lesions tend to have low signal intensity. Lesions with less fat tissue in the marrow show decreased signal intensity. Cavernous hemangioma is typically enhanced after administration of gadolinium and shows high signal intensity on T2-weighted MRI due to slow-flowing blood. Histologically, hemangioma is classified as one of three types: cavernous, capillary, or mixed. Cavernous hemangioma is composed of a cluster of large, dilated blood vessels separated by fibrous tissue, while capillary hemangioma lacks fibrous septa and has smaller vascular lumina. Most calvarial hemangiomas are the cavernous type, whereas vertebral hemangiomas are most frequently of the capillary type. The bony trabeculae within this tumor are thought to be the result of osteoclastic resorption and osteoblastic activity by the growing vascular tumor [5]. The treatment of choice for intraossoeus hemangioma is en bloc resection with an adequate normal bone margin, and the bony defect can be reconstructed by various methods. Other therapeutic methods include curettage, radiotherapy, and embolization. However, partial removal is likely to be followed by recanalization. Radiotherapy can reduce the tumor volume and has demonstrated symptomatic improvement, but it has the risk of radiation-induced carcinoma. Therefore, radiation therapy should be reserved for cases that are unresectable or have a residual tumor from subtotal resections. Curettage is not a good treatment modality because it can produce excessive bleeding and shows a high recurrence rate.
In conclusion, intraosseous cavernous hemangioma is a rare bone tumor in the calvarium and demonstrates similar radiologic findings to other bone tumors. It can thus be confused with other bone tumors. Therefore, the diagnosis is frequently made in the operative field by its hemorrhagic features and confirmed by histologic examination. The treatment of choice is surgical resection with an adequate normal bone margin.
Notes
This article was presented at the 70th Congress of The Korean Society of Plastic and Reconstructive Surgeons on November 9-11, 2012 in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.