Reconstruction of Abdominal Wall of a Chronically Infected Postoperative Wound with a Rectus Abdominis Myofascial Splitting Flap
Article information
Abstract
Background
If a chronically infected abdominal wound develops, complications such as peritonitis and an abdominal wall defect could occur. This could prolong the patient's hospital stay and increase the possibility of re-operation or another infection as well. For this reason, a solution for infection control is necessary. In this study, surgery using a rectus abdominis muscle myofascial splitting flap was performed on an abdominal wall defect.
Methods
From 2009 to 2012, 5 patients who underwent surgery due to ovarian rupture, cesarean section, or uterine myoma were chosen. In each case, during the first week after operation, the wound showed signs of infection. Surgery was chosen because the wounds did not resolve with dressing. Debridement was performed along the previous operation wound and dissection of the skin was performed to separate the skin and subcutaneous tissue from the attenuated rectus muscle and Scarpa's fascial layers. Once the anterior rectus sheath and muscle were adequately mobilized, the fascia and muscle flap were advanced medially so that the skin defect could be covered for reconstruction.
Results
Upon 3-week follow-up after a rectus abdominis myofascial splitting flap operation, no major complication occurred. In addition, all of the patients showed satisfaction in terms of function and esthetics at 3 to 6 months post-surgery.
Conclusions
Using a rectus abdominis myofascial splitting flap has many esthetic and functional benefits over previous methods of abdominal defect treatment, and notably, it enabled infection control by reconstruction using muscle.
INTRODUCTION
A chronic wound usually refers to a wound which, despite appropriate treatment, shows no anatomical or functional improvement and lasts more than 4 weeks [1].
If a chronic wound is accompanied by infection, a great deal of time and effort are necessary for treatment because the normal wound healing mechanism could be hindered and the inflammatory reaction could be distorted. Also, if the chronic wound is located in the abdomen, the wound could get deeper and thus an anterior wall defect or peritonitis could develop.
Chronic wounds cause a vicious cycle which leads to repetitive operations, increased hospital days, decreased patient immunity and increased possibility of infection, in that order. A vicious cycle like this, therefore, must be broken by inhibition of the infection in a timely fashion.
In case of an abdominal chronic wound, it is not easy to release the skin tension when suturing the skin by using a primary suture, and it is also difficult to avoid the increase of fascial retraction or adherence. Furthermore, when tension rises on the skin due to a primary repair, ischemic changes could occur in the skin. Furthermore, if infection caused by necrosis invades underneath the peritoneum, chronic wound infection could develop.
For this reason, if chronic wound infection is treated by surgery using a myofascial splitting flap, bacterial clearance would be increased due to the ample blood supply from vessels distributed on the muscle pedicle, and also the chronic infected wound would be effectively controlled due to an increased metabolic turnover rate of the tissue [2,3].
In this study, chronic postoperative abdominal wounds were treated using a rectus abdominis myofascial splitting flap unlike in previously reported studies using other methods, and the infection was effectively controlled and the recovery was uneventful as a result.
METHODS
Subjects
Five patients who were referred to our department from January 2009 to February 2012 for collaborative treatment because of open wounds after gynecological surgery were chosen as subjects.
Their age ranged from 28 to 59 and they were all female patients. Two had ovarian rupture, 2 had cesarean section complications, and 1 had received a uterine myoma operation. Ongoing redness, swelling, tenderness on pressure, and exudation occurred after surgery. They had been referred for collaborative treatment because there was no significant improvement even though wound dressing had been performed for 1 to 2 weeks. The results of bacterial culture carried out before treatment showed 2 cases of Escherichia coli and 3 of Klebsiella pneumonia (Table 1).
All the stitches on the open wound site were removed afterwards and wound dressing including irrigation 2 to 3 times per day was performed for 2 weeks. After that, no particular bacterium was cultured in any of the 5 cases when a bacterial culture of the wound was done. Later on, surgery was performed (Fig. 1).
Method
The operations were performed under general anesthesia for all patients.
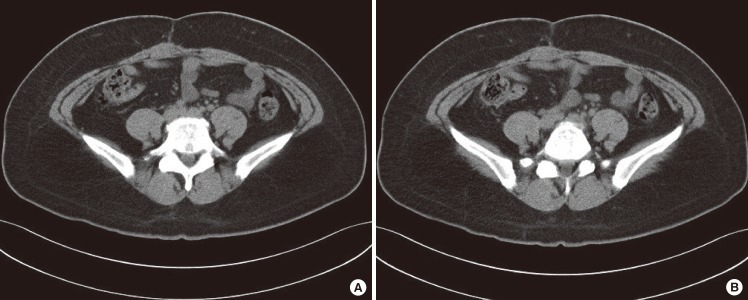
A visible infection was extensively affecting the rectus abdominis muscle and the area just superior to the peritoneum. Including partial healthy tissue, debridement was performed on the infected wound with an effort to preserve the rectus abdominis muscle as much as possible (Fig. 2A, B).
Intraoperative photograph: surgical specimens after debridement
(A) The infected wound extended from the skin to part of the rectus abdominis muscle. (B) Intraoperative view of the abdominal defect that developed after debridement. (C) Without damaging the arteries, the rectus abdominis muscle is being split. (D) The abdominal defect was reconstructed by turning over the bilateral rectus muscle flaps medially. (E) The arteries inside the rectus abdominis muscle are being preserved as much as possible. Sutures were conducted with the flaps overlapping each other.
Enough of the skin and underlying adipose tissue were separate from the anterior rectus sheath to cover the middle abdominal wall defect. The size of the middle abdominal wall defect was measured accurately, and the area for incision was chosen in order for the creation of a splitting flap.
Alongside both lateral borders within the determined range, a longitudinal incision which split the rectus abdominis muscle along with the anterior rectus sheath was made to elevate the rectus abdominis muscle, which could cover the defect size. When doing so, confirmation using both visual inspection and Doppler was performed to preserve the deep inferior epigastric artery, and functional dissection alongside the muscle layer was conducted so that the perforating arteries which branched from the deep inferior epigastric artery could be preserved.
Through these efforts, not only unfolding of the muscle but also minimization of artery damage were achieved (Fig. 2C). Bilaterally elevated rectus abdominis muscles including the anterior rectus sheath were turned over to be positioned on the midline (Fig. 2D). The bilateral muscles were positioned to overlap each other without tension, and then two layers were sutured by the through-and-through suture technique (3-0 polyglactin) (Fig. 2E). On the bilateral ends of the overlapped part, a tension-free interrupted suture was performed to prevent the ends from moving (Fig. 3). The tension-free suture was possible because the defect area was able to be sufficiently covered by the split muscles, and thus a strong suture that could possibly cause infection or muscle ischemia was not necessary.
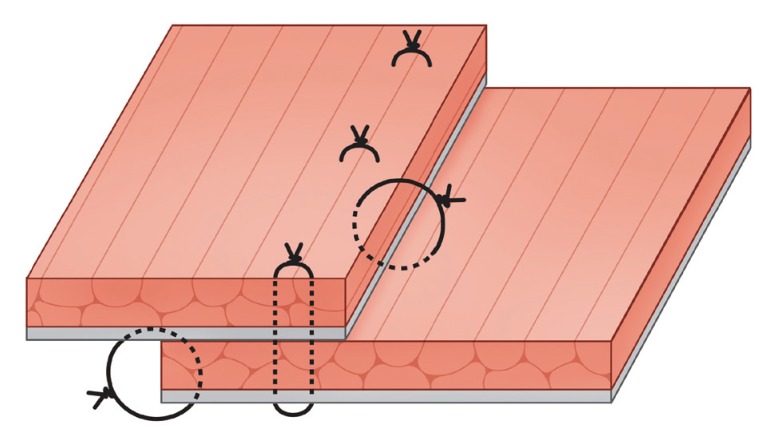
Overlapped rectus abdominis muscle and anterior rectus sheath unit
Instead of a strong suture that could cause infection or muscle ischemia, a through-and-through suture was made with the muscles overlapped. The black color is the anterior rectus sheath, and the red color is the rectus abdominis muscle.
One drain was placed in the inferior part, and skin closure was performed by interrupted stitches (Fig. 4).
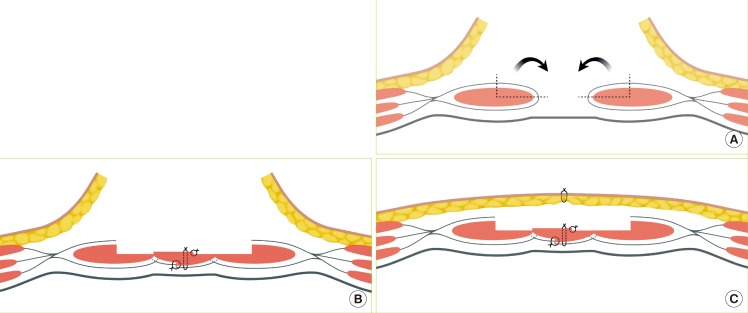
Schema of surgical methods
(A) A portion of the rectus abdominis muscle that could sufficiently cover the defect size is elevated along with the anterior muscle sheath, and then the elevated muscle is turned over toward the midline, by turning over the anterior rectus sheath along with the rectus abdominis muscle, a horizontal mattress suture was made. (B, C) On the bilateral ends of the overlapping part, an interrupted suture was made, after suturing was completed, the skin and subcutaneous tissue were sutured after confirming circulation by visual inspection or Doppler.
RESULTS
The patients who underwent surgery were all females and their mean age was 41.2 years. They were referred to our department with the chief complaint of a chronically infected abdominal wound after receiving a gynecological operation due to ovarian rupture (n=2, 40%), cesarean section complications (n=2, 40%), or uterine myoma (n=1, 20%).
The average time it took from gynecological surgery until the referral due to infectious signs was 14.7 days (range, 10 to 21 days), and it was 35.4 days (range, 28 to 42 days) from the original treatment until undergoing surgery in our department. The mean operative time was 140 minutes (range, 120 to 170 minutes), and all of the patients received postoperative recovery treatment in general hospital rooms with an average hospital stay of 15.3 days (range, 12 to 20 days).
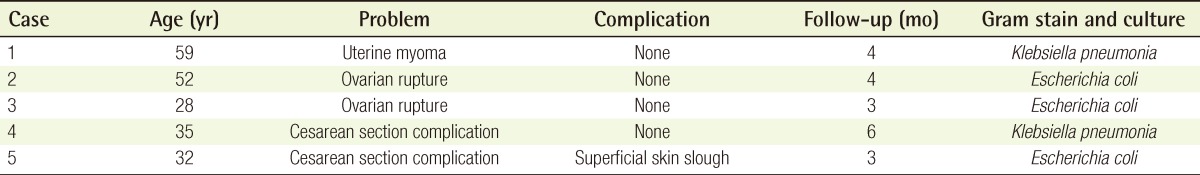
In all 5 cases, reconstruction of the abdominal defect due to a chronically infected wound was performed with a rectus abdominis myofascial splitting flap. Follow-up care was provided for an average of 14 months (range, 3 to 6 months), and 1 case showed superficial skin slough development. However, the skin slough problem was resolved by a simple skin revision suture. Other than that, no particular complications such as recurrence of infection due to myofascial flap use, necrosis of the flap, avulsion of the suture, or instability of the abdominal wall appeared, and satisfactory outcomes were obtained in all cases (Fig. 5).
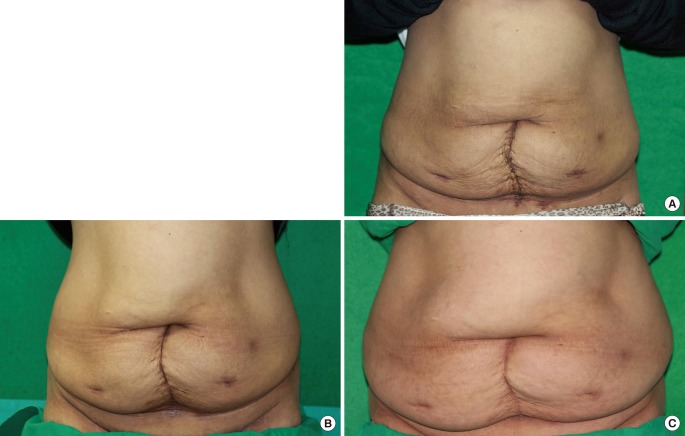
Case 1: postoperative photographs
(A) One month postoperative photograph of abdominal wound, (B) 4-month postoperative photograph of the abdominal wound, (C) 10-month postoperative picture of abdominal wound.
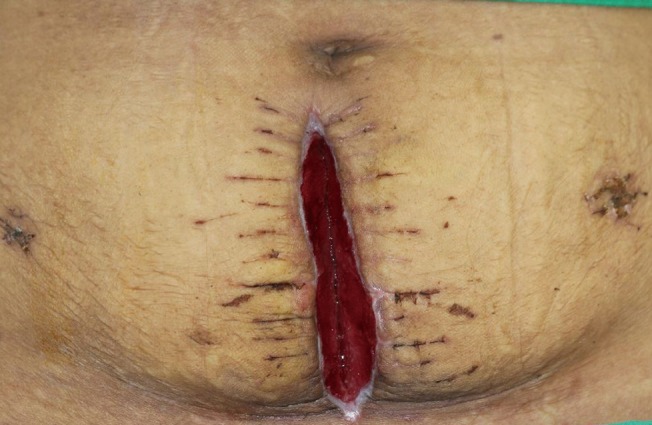
An abdominal computerized tomogram (CT) was taken at least 10 months after surgery to confirm whether the split rectus abdominis muscle was well maintained or whether a hernia due to weakness of the abdominal wall occurred. As a result, it was confirmed that weakening of the abdominal wall after surgery and abdominal hernia did not occur (Fig. 6).
DISCUSSION
Normal wound healing goes through a blood coagulation phase, inflammatory phase, proliferation phase, and remodeling phase in that order, and it usually takes 3 weeks for the wound to be completely covered by epithelium. However, if normal wound healing fails for some reason and the wound remains unhealed for more than 4 to 8 weeks, it is called a chronic wound [4,5].
When a chronic wound is accompanied by infection due to internal or external factors, many difficulties arise in wound healing because normal wound healing is inhibited and the inflammatory reaction is distorted. A great deal of extra time and effort beyond palliative dressing methods are needed for a wound like this to heal properly, and in some cases surgery cannot be attempted because of accompanying factors or cases where systemic antibiotic treatment is not effective [6].
Therefore, method is necessary that will reduce additional tissue injury and infection while creating the appropriate environment for wound healing by controlling infection so that the distorted healing process is corrected.
In the cases described here, the abdominal wall defect was caused by chronic wound infection that developed postoperatively. The abdominal wall comprises stratified layers of skin, superficial fascia, muscle, aponeurosis, properitoneal fat, transverse abdominal muscle fascia, and peritoneum. The superficial fascia is composed of a superficial fat layer, Camper's fascia, Scarpa's fascia, etc., and it is connected with the tensor fascia lata. As explained above, the abdominal wall is composed of various complex layers. Because the abdominal wall protects important structures in the abdominal cavity, abdominal wall defects could cause serious life-threatening complications for any number of reasons.
Therefore, abdominal wall defects should be reconstructed with maximum urgency. Reconstruction methods can vary widely depending on the depth and location of the defect. In case of a simple skin defect where the scope of the abdominal wall defect is not wide, reconstruction could possibly be done with a skin graft only, and in case of a skin defect involving thick subcutaneous adipose tissue-for example, a skin defect formed after dissection of a skin tumor or burn scar, reconstruction could be performed by using a local skin flap rather simply and esthetically as well. If expected size of the defect is too large to be reconstructed by a local flap, a tissue expander could be used. If the defect reaches up to the muscle or peritoneum, reconstruction should be done by a myocutaneous flap alone or, if sufficient anatomical recovery could not be gained by a skin flap alone, a myocutaenous flap and skin graft together should be used [7].
Further information on abdominal wall defects is available from previous studies.
Vacuum-assisted closure (VAC, Kinetic Concepts Inc., San Antonio, TX, USA), which was introduced by Argenta in 1997, is used as an effective adjunctive method for wound healing, and it improves the local blood supply by putting even pressure to a wound, increases neovascularization, decreases the possibility of wound infection by reducing the bacterial count, boosts formation of granulation tissue, and also removes excess interstitial fluid, which includes inhibiting factors against wound healing; thus, it is effective in swelling relief and wound healing [8].
However, unstable skin, necrotic tissue, and severe infection are contraindications in the use of VAC. Also, the use of VAC in chronically infected wounds causes a significant increase in treatment costs. In 1999, Philbeck et al. [1] reported a case in which a VAC device was used, and it turned out that the treatment lasted for 97 days and cost US$14,546 (US$149/day) in total. DeFranzo et al. [9] reported that if VAC were used for treating bone exposure wounds in a lower extremity, it would cost approximately US$100/day. The burden of an expense like this is judged to be a factor that inhibits the appropriate use of VAC [9].
Also, another problem lies in the fact that moving patients is inconvenient for both the medical team and the patient because the heavy VAC device must be placed next to the patient during vacuum treatment.
Therefore, as an alternative, the rectus abdominis myofascial splitting flap that was used in this study has many advantages. It is not a mere adjunctive treatment measure, and one single surgery made abdominal wall reconstruction possible, while decreasing the hospital stay and promoting rapid recovery at a low cost compared with VAC treatment.
In 2007, an abdominal wall defect was reconstructed using a bilateral anterior rectus abdominis sheath turnover flap, introduced by Kushimoto et al. [10]. In this method, a longitudinal incision is made alongside the lateral edge of the anterior rectus sheath so that it becomes mobilized, and skin closure is carried out after medial approximation. Through this method, early fascial closure could be achieved and also skin grafting and subsequent abdominal wall reconstruction became less necessary than before. Thus, it became one of the important techniques of early management of open abdominal wounds [10]. However, this method cannot easily be applied to wounds with chronic infection. In particular, in the case of a wound that includes the midline of the abdomen, because muscle other than the linea alba does not exist in this area, infection may easily develop if the defect is supplemented by an anterior rectus abdominis sheath only, and this may not be sufficient in terms of reinforcing the abdominal wall.
Besides, the linea alba forms a band while reaching the xiphoid process superiorly and reaching the symphysis pubis inferiorly, and it forms a collagen fiber band formed by cross fusion of the external oblique abdominis muscle, internal oblique abdominis muscle, and aponeurosis of the transverse abdominis muscle, and this plays a key role in the stability of the abdominal wall [11].
If infection occurs in the linea alba due to dissemination of the chronic wound infection, an effort to reconstruct the wound area using only an anterior rectus sheath will not help control infection, and it actually could possibly turn out to become a new culture medium for infection. Furthermore, it will not be able to prevent the infection from being transmitted to the peritoneum because the linea alba has been removed.
In this study, each defect was reconstructed not only with anterior rectus abdominis sheath but also with rectus abdominis muscle. By doing so, the possibility of insufficient reinforcement of the abdominal wall was reduced, and stability could be guaranteed both at both the original muscle location and the defect site because only part of the muscle was used. In addition, the flap could function as a barrier against infection because the anterior rectus abdominis sheath can supplement the peritoneum by being located underneath the rectus abdominis muscle.
In 2008, Chuo and Thomas [12] reported a study in which absorbable mesh was used along with topical negative pressure therapy in order to supplement an abdominal wall defect, and in 1986, Dayton et al. [13] reported on another study in which absorbable mesh was used to reinforce the abdominal wall after a contaminated abdominal wall defect was treated. In both studies, it was reported that the method in which prosthetic material was used to supplement the abdominal wall enabled static support without tension and enabled prevention of complications such as infection.
However, neither of these studies included long-term follow up. In another study on long-term complications of a case in which prosthetic material was used to supplement the abdominal wall, it was reported that the prosthetic material had a supple and elastic nature, and its grainy texture gripped tissue and thus long term complications occurred in which a rapid fibroblastic response around tissue was caused.
It was also reported that monofilament or multifilament braided mesh could not exclude bacteria although it excluded macrophages, and this caused infection, foreign-body granuloma, and sinus tract formation [14].
In this study, the possibility of postoperative recurrence or secondary contamination was reduced by excluding the use of prosthetic material because using prosthetic material on a contaminated wound could cause the above-mentioned complications. The risk of problems such as attenuation of the abdominal wall was reduced by anterior rectus sheath turn-over and muscle splitting.
In 1990, Ramirez et al. [15] used a component separation operation in which the aponeurosis between the rectus abdominis muscle and external abdominal oblique muscle was disconnected and the posterior rectus sheath, which was inferior to the rectus abdominis muscle, was separated from the muscle when a large abdominal wall defect existed. This method enabled preservation of the arteries because an incision in the rectus abdominis muscle was not made, and thus had the advantage that the original anatomy itself was able to be used. However, the thickness of the rectus abdominis could decrease and some degree of tension was inevitable because muscle had to be extended enough to cover a large defect area after disconnecting the aponeuroses that were attached to the rectus abdominis muscle and posterior rectus sheath. In this study, what caused the abdominal wall defect was chronic infection that had disseminated from the skin. For this reason, applying the method by Ramirez could have made infection control difficult because the wound area and anterior rectus sheath come into contact with each other, and if the posterior rectus sheath is disconnected, the efficacy of the barrier that could prevent infection will decrease when the infection is disseminated to the peritoneum.
As opposed to this, the rectus abdominis myofascial splitting flap that was used in this study enabled infection control by making the wound area and part of the muscle come into contact with each other, and the anterior rectus sheath that was turned over could function as another barrier for stopping infection from being disseminated. Although the thickness of the rectus abdominis muscle is decreased, which is a disadvantage compared to the method suggested by Ramirez et al. [15], it was confirmed that the abdominal wall was not weakened, as shown by a CT 10 months later.
If a muscle component of the abdominal wall is partially separated, it could make the flap reach out farther than when the whole abdominal wall is moved as one unit, and if a muscle with contractibility is used for suturing the abdominal wall, it is more resistant to stress or strain than when prosthetic material is used, and this could lower the probability of re-infection by a foreign substance and could increase bacterial clearance through sufficient blood supply while maintaining the nerve and vessel supply. Because the superior and inferior epigastric vessels among these vessels travel superiorly and inferiorly to the rectus abdominis muscle, when the method suggested in this study where the muscle has to be split in the direction of the muscle and the vertical direction as well is performed, an abundance of blood vessels could also be gained following the unfolding of the muscle.
In other words, if the rectus abdominis muscle flap that was suggested by this study were used, complete recovery could be gained by one single surgical treatment compared with other methods that require multiple operations, thus reducing the duration of hospital stay and cost, while minimizing the patient's pain. Furthermore, this method reinforces the abdominal wall and reduces the possibility of secondary infection. Therefore, it can be said that this method is one of the effective methods with many benefits over previous methods among the those for surgical treatment of abdominal wall defects caused by postoperative chronically infected abdominal wounds.
Notes
No potential conflict of interest relevant to this article was reported.