Basal Cell Carcinoma Presenting as a Hypertrophic Scar
Article information
Basal cell carcinoma (BCC) is one of the most common skin malignancies. Its well-known etiologic factors include ultraviolet (UV) exposure, ionizing radiation, arsenal exposure, and genetic predisposition. Moreover, its etiological association with trauma or scar tissue has also been documented [1-3].
A 39-year-old woman visited us with the chief complaint of a hypertrophic scar occurring in the right supratip region (Fig. 1A). The patient had no past history of having abnormal symptoms or diseases in the right supratip region, but presented with a 10-month history of acne-like pustules. The patient also stated that the inflammation waxed and waned as the scars were frequently irritated during wound healing. Thereafter, residual scars gradually protruded and were suggestive of hypertrophic scarring, for which the patient visited us to undergo a scar revision. On physical examination, the patient had a round, protruding, solid, hard nodular lesion, measuring approximately 0.7 cm×0.7 cm in size. Grossly, the nodule showed a well-defined margin and a smooth surface, which was accompanied by depigmentation as well as mild capillary dilatation. There were no such findings as erosion, eschar, or bleeding. The patient also had no subjective symptoms such as pruritus or pain. Clinically, the patient was tentatively diagnosed with hypertrophic scarring occurring after recurrent episodes of trauma. First, considering that the patient was a young unmarried woman, we wished to minimize the postoperative nasal deformity and scar formation. We therefore decided to induce epithelialization following the tangential resection of the nodule. Following a resection of the nodule, however, we could not completely rule out the possibility of a tumor because it is well established that skin tumors commonly occur on the nose. We therefore performed a histopathologic examination, and the patient was diagnosed with nodular BCC (Fig. 2). The patient then underwent a complete resection of the tumor at a subcutaneous depth involving a 2-mm safe margin. The site of the defect was reconstructed using a bilobed flap (Fig. 1B). Currently, at 18 months postoperatively, the patient has been undergoing regular follow-up. The patient had no recurrence, and cosmetic outcomes were satisfactory (Fig. 1C).
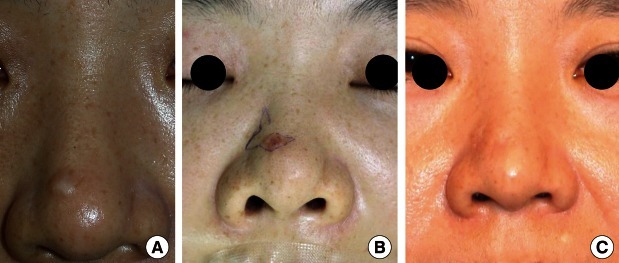
Clinical photos. (A) Preoperative view. A well-defined, 0.7 cm×0.7 cm round, skin-colored nodule on the nasal dorsum. (B) Intraoperative view. Surgical design using a bilobed flap. (C) Postoperative view. Good cosmetic results 12 months after excision.
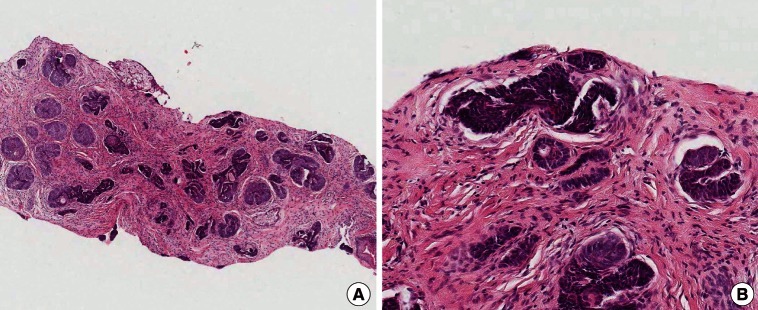
Histologic findings. (A) Histologic image showing a tumor mass composed of nests of basaloid cells (H&E, ×4). (B) Tumor nests showing peripheral palisading nuclei and peritumoral fibrosis (H&E, ×200).
BCC occurs frequently in the head-and-neck regions including the face; common sites of occurrence include the nose and eyelid. In addition, most cases occur in adults aged 40 years or older, and they also show a predilection toward body areas that are vulnerable to long-term sunlight exposure. Presumably, this might be because the long-term, excessive exposure to physicochemical irritants might induce the occurrence of skin malignancies [2].
In 1828, Jean-Nicholas Marjolin et al. first described the possible role of trauma in the pathogenesis of skin malignancies. Since then, several authors have reported the malignant transformation of a wound or scar tissue. "Marjorlin's ulcer" was originally coined to describe the cancer originating from a burn injury scar. However, its usage has since been extended to describe all malignancies arising from scar tissue [3].
In 1986, Noodleman and Pollack [3] reported that there was a relationship with trauma in 7.3% of 1,774 cases of BCC. More recently, Ozyazgan and Kontas [1] reported that trauma and scar tissue play a role in the pathogenesis of BCC. In Korea, two cases of BCC have been reported to have arisen from trauma [4]. To date, however, few reports have addressed this subject compared with about the number of reports on squamous cell carcinoma arising from scar tissue. The length of time that elapses from the onset of trauma until a tumor occurs can vary, ranging from several weeks to several decades. Many types of trauma have been documented as causes of BCC, such as burn injury scars, vaccination sites, chicken pox scars, tattoo sites, sharp or blunt physical injury, skin exposed to polycyclic aromatic hydrocarbons, lupus vulgaris, chronic stasis ulcers, sites of cold injury, site of hair transplantation, lesions of epidermolysis bullosa, colostomy sites, dog bites, electrical burns, and gunshot wounds [1-5].
Little is known about the pathophysiology of the malignant transformation of scar tissue [1,3]. However, reports suggesting various types of involvement of trauma in the pathophysiology of BCC have included the following: 1) The BCC incidentally occurs with no etiological association with the trauma. BCC is a very common disease entity. Therefore, based on the recognition of major skin trauma or wounds, patients spontaneously consider the BCC to have occurred in association with the trauma. However, there is no actual correlation between the two phenomena [3].
2) The skin becomes more vulnerable to UV exposure because of the atrophy and decreased perfusion in the trauma-induced scar tissue. Thus, the nutritional supply is compromised, which eventually leads to a lack of ability to repair DNA damage [3].
3) With chronic irritation to the damaged skin tissue, malignant transformation occurs. The damaged skin tissue releases toxins that may cause cellular mutation. Chronic wounds exposed over the long-term to these toxins become more vulnerable to malignant transformation [5].
4) In cases of tumors arising from dense scar tissue, immunological privilege prevents lymphocyte infiltration. This interferes with the immune surveillance system. Thus, the tumor can protect itself from human defense mechanisms until its growth reaches a significant level [3].
5) In the presence of trauma, the epidermal cells are implanted into the dermis. This triggers the occurrence of foreign body reactions in the dermis, alters the normal wound healing activity of the tissue, and renders the tissue more vulnerable to damage [5].
In cases of BCC arising from trauma or scar tissue, the degree of malignancy is not relatively higher than that due to other causes [1,3]. Therefore, standard treatment modalities are performed for the management of BCC.
Our case highlights that BCC may also occur even after the trauma. Therefore, clinicians must rule out the possibility of malignant transformation when they examine patients with traumatic scars located on body surfaces that are vulnerable to sunlight exposure.
Notes
This study was presented at the 70th Congress of the Korean Society of Plastic and Reconstructive Surgeons on November 9-11, 2012, in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.