Writing an Evidence-Based Articles in Plastic Surgery Field
Article information
Many beginning writers, especially medical residents, feel difficulties in writing journal articles. However, illogical and unscientific papers are not accepted for publication even if their topics are creative and interesting. The authors should do their best to convince reviewers and readers of their opinions using evidence through reasoning, explanation, and data interpretation.
The aim of this article is to review evidence-based medicine (EBM) and reporting guidelines and to assist authors in composing plastic surgery papers with a logical argument.
The concept of EBM was initially suggested in 1979 by Archie Cochrane, who was a British epidemiologist. Since then, the trend of modern medicine has been toward completely evidence-based decision making [1]. Recently, the American Society of Plastic Surgeons (ASPS) and the journal Plastic and Reconstructive Surgery (PRS) have been actively working to build a foundation for EBM in the plastic surgery field [2].
In August 2010, various plastic surgery researchers and journal editors had a conference in Colorado Springs, US for the integration of EBM into the field of plastic surgery. At the conference, it was decided that the level of evidence and a visual icon of the evidence pyramid would be displayed at the end of the abstract of every article, except some that could not be rated, with classification of the research into one of these categories: diagnostic, therapeutic, prognostic, and risk. This decision has been in effect in PRS since January 1, 2011 [3]. Among the evidence levels assigned in PRS, level 1 represents the highest or strongest level of evidence, while level 5 is the lowest. The types of studies in level 3 include retrospective cohort studies and case control studies. The case series is included in level 4 [4]. The average level of plastic surgery articles submitted to PRS is 3.2 and most are between 3 and 4.
The reasons for the relatively low levels in the field of plastic surgery are that each patient has highly variable requirements and some cases are rare due to the nature of plastic surgery, and there are diverse approaches to treating a given diagnosis [5]. To overcome these limitations, PRS estimate the level of evidence on all articles according to subsections. And PRS recommends that researchers aim to produce prospective cohort studies, which are in level 2, and eventually even level 1, randomized controlled studies, even though researchers typically start out by publishing level 3, 4, or 5 articles [3,6].
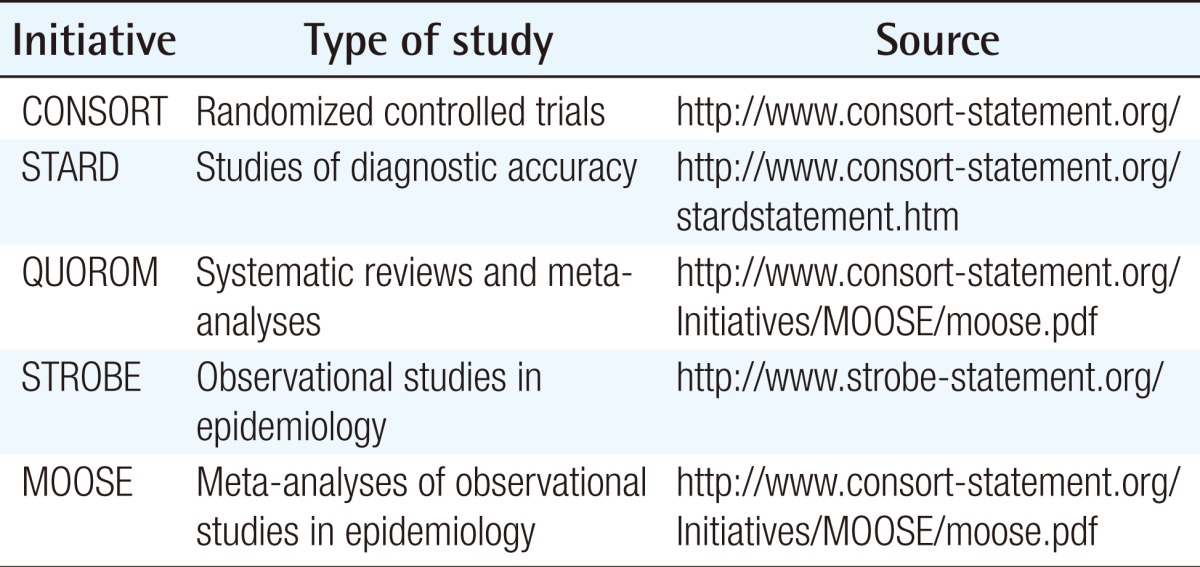
As the Korean Society of Plastic and Reconstructive Surgeons has revised the submission guidelines for this journal, the Archives Plastic Surgery (APS), it is now recommended that articles be written using five reporting guidelines, which are provided in the appendix, to improve the evidence level (Table 1).
Because there are many articles at levels 3, 4, and 5 due to the nature of the plastic surgery field, referring to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), which is suitable for observational studies, would be useful. Although STROBE is a guideline developed for observational studies in epidemiology, it is also applicable to plastic surgery. In particular, if the STROBE checklist were applied to retrospective cohort studies, case control studies, case series, and case reports, important information that should be in the articles would not be omitted and the transparency of research results would be achieved [7]. On their website (www.strobe-statement.org), the STROBE research group also publicly announces the latest research results on guidelines, and revisions to the guidelines under ongoing development by researchers and editors. In addition, checklists are provided free of charge to readers in PDF and Word formats (Table 2).
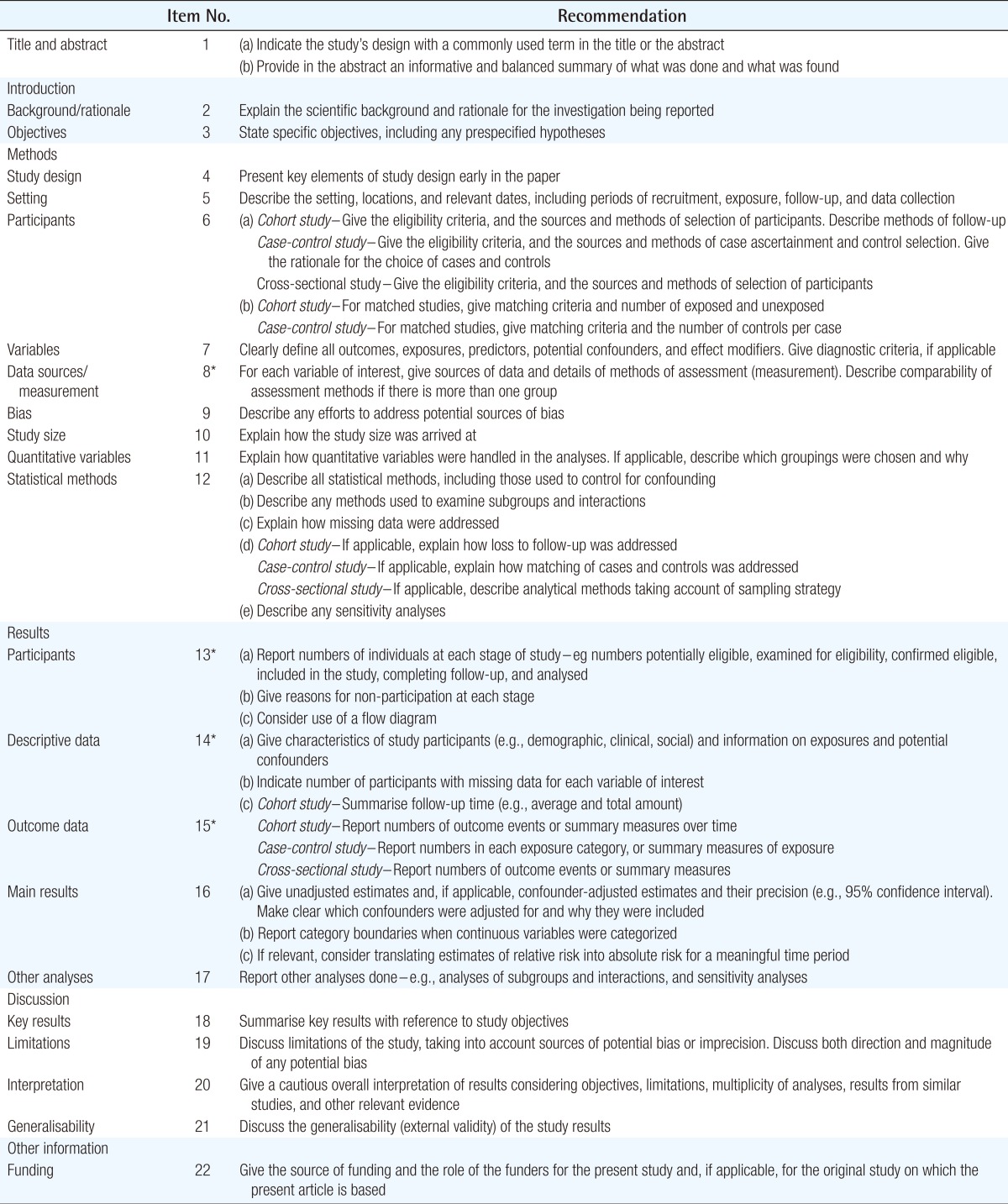
Strengthening the Reporting of Observational Studies in Epidemiology Statement.checklist of items that should be included in reports of observational studies
If and when you do a game, you need to know the rules first. Writing an article is much easier when you know the rules and have a set of guidelines to follow. It is hoped that the resources provided here will assist the readers of APS to become successful researchers and authors.
Notes
No potential conflict of interest relevant to this article was reported.