Robotically Assisted Microsurgery: Development of Basic Skills Course
Article information
Abstract
Robotically assisted microsurgery or telemicrosurgery is a new technique using robotic telemanipulators. This allows for the addition of optical magnification (which defines conventional microsurgery) to robotic instrument arms to allow the microsurgeon to perform complex microsurgical procedures. There are several possible applications for this platform in various microsurgical disciplines. Since 2009, basic skills training courses have been organized by the Robotic Assisted Microsurgical and Endoscopic Society. These basic courses are performed on training models in five levels of increasing complexity. This paper reviews the current state of the art in robotically asisted microsurgical training.
INTRODUCTION
Developed in the 1960s, microsurgery is a surgical technique using optical magnification that allows the microsurgeon to perform delicate movements which are difficult or impossible using the naked eye. Over the last few years, microsurgery has seen two major technical advances: supermicrosurgery [1] and telemicrosurgery [2]. The latter, telemicrosurgery or robotically assisted microsurgery can be defined as the technique of microsurgery that uses robotic telemanipulators to scale down surgical gestures or movements. The da Vinci robot (Intuitive Surgical Inc., Sunnyvale, CA, USA) is the only medical robot currently available on the market for robotically assisted microsurgery.
Several international groups, including the Robotic Assisted Microsurgical and Endoscopic Society (RAMSES, http://www.roboticmicrosurgeons.org), have reported their experiences in various disciplines such as urology, plastic surgery, Ear, Nose, Throat (ENT) surgery, ophthalmology, neurosurgery, hand surgery, and peripheral nerve surgery [3]. RAMSES not only aims to promote microsurgery with robotic manipulators, but also aims to develop a new concept: endoscopic microsurgery. It combines the properties of microsurgery, endoscopic surgery, and telesurgery. This would not only allow magnification of the view of the operating field, but also enable microsurgeon to scale down the gestures or movements of the operator's hands, while taking a minimally invasive approach.
While telemicrosurgery is still in its infancy, RAMSES has organized telemicrosurgery classes since 2009. The purpose of this paper is to describe the models used for training, evaluation methods, and the organization and proceedings of basic and advanced telemicrosurgery training.
THE DA VINCI ROBOT
The only surgical telemanipulator currently available on the market is the da Vinci robot (Intuitive Surgical Inc.). It consists of three components: a mobile instrument cart with four articulated arms, an imaging cart, and a console for the surgeon to control the robotic arms (Fig. 1). The mobile cart contains four articulated robotic arms, three of which carry surgical instruments and a fourth arm that manipulates the digital stereoscopic camera to visualize the surgical field. Each of these arms has multiple joints providing three-dimensional movement of the surgical instruments and optics. The surgical tools have articulation that provides intracorporeal range of movement to 360°, called "EndoWrist" technology. The tools available vary: dissecting forceps, scissors, scalpel, spreaders, etc. The fourth arm, which controls the optics, has a stereoscopic, high definition, endoscopic camera. The stereoscopic camera lens comprises a video imaging column similar to that used in conventional laparoscopy or arthroscopy and two light sources and dual stereoscopic cameras for three-dimensional vision with progressive magnification up to 12 to 15 times. The surgeons' console is equipped with an optical viewing system, two telemanipulation handles, and five pedals. The optical viewing system, called the stereo viewer, offers a three-dimensional view of the operating field and displays text messages and icons that reflect the status of the system in real time. The two telemanipulation handles allow remote manipulation of the four articulated robotic arms. In its latest version, the da Vinci SI, the robot is equipped with 2 surgeon consoles to allow for the simultaneous use with two operators: the primary robotic surgeon and a surgical assistant. In this mode, the 3 robotic arms can be utilized at the same time by the two operators.
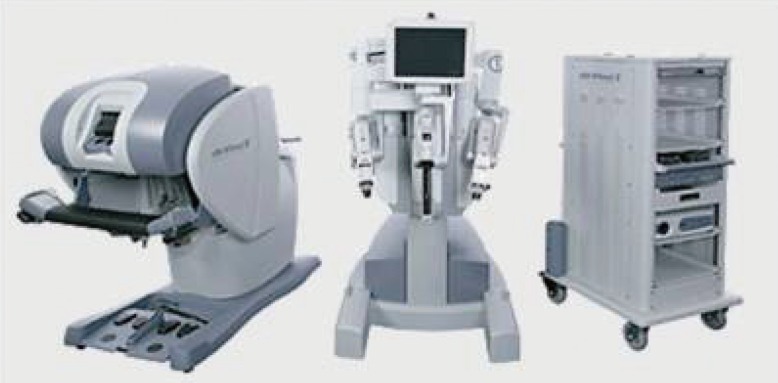
Da Vinci Robot
The robot da Vinci SI contains three parts (from right to left): a mobile cart with four articulated arms, an imaging cart, and a console for the surgeon to control the robot arm (reprinted with permission from Intuitive Surgical, Sunnyvale, CA, USA).
Five properties of the da Vinci robot are essential in telemicrosurgery. The optical magnification of the operating field is obtained by the optical and digital magnification of the stereoscopic camera. The suppression of physiological tremor improves the quality of surgical movements. The scaling down of surgical movements improves accuracy by reducing the surgeon's movements by a factor of 3 (position "fine") or 5 (position "extra-fine"). The ergonomic design of the surgeons' console is very useful in microsurgery because it improves the comfort of the surgical movements by simplifying the motion. The possibility of minimally invasive surgery allows the microsurgeon to work in unique operative fields with minimal cutaneous incisions.
Robotic telemanipulators have often been criticized for not having tactile feedback. In reality, it has been clearly demonstrated that force or tactile feedback is not absolutely necessary in microsurgery: first, because the range of motion in the microsurgical field is minimal, and second, because the perception of a 9/0 and 10/0 nylon yarn voltage is at the limit of human physiology [4]. Successful microsurgery with suture-assisted microsurgical robots has already been reported [5]. In addition, a new robotic platform, the Amadeus telemanipulator (Titan), that will be available soon, will be equipped with tactile feedback. It is not difficult to imagine that, in the future, there will be a robot able to replicate microsurgical maneuvers with tactile feedback force, possibly enabling endoscopic supermicrosurgery [1].
TRAINING MODELS
Among the models currently available in conventional microsurgery, there are nonliving non-biological models (latex, silicone, Gore-Tex, PracticeRat), nonliving biological models (artery of the chicken wing, pig's feet, placenta) and living models (mouse, rat, rabbit) [6,7]. The ideal model would meet the following specifications: inexpensive, easily available, similar to the biological tissue, without ethical issues [8]. No model perfectly fulfills all these specifications and full education in telemicrosurgery must have several levels of increasing complexity, the ability to address technical challenges, and applicability to varying microsurgical procedures.
The first level is to become familiar with the robot and master the basic skills of telemicrosurgery. This can be done either by means of an analog simulator such as plastic rings (Fig. 2) or by means of a virtual simulator. Several simulators are available on the market: Mimic, Ross [9], dV-Trainer [10], and da Vinci skills simulator [11]. The virtual simulators assess students' performances in terms of time to complete the exercise, gesture accuracy, missed targets, instrument collision, drops, etc. This is one reason why virtual simulators allow for highly efficient self-teaching (Fig. 3).
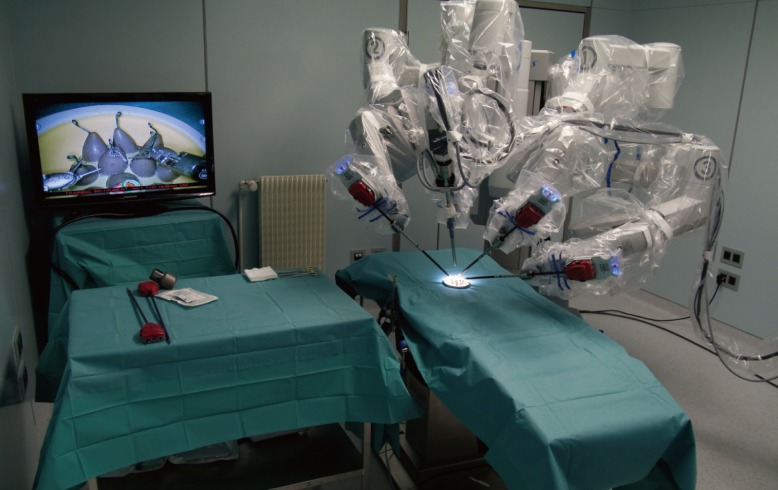
Robot set-up
Installation of a robot da Vinci SI for level 1 training. Plastic rings must be manipulated and moved from one display stand to the other. No measure of performance is possible with this model, except the time of realization of the task.
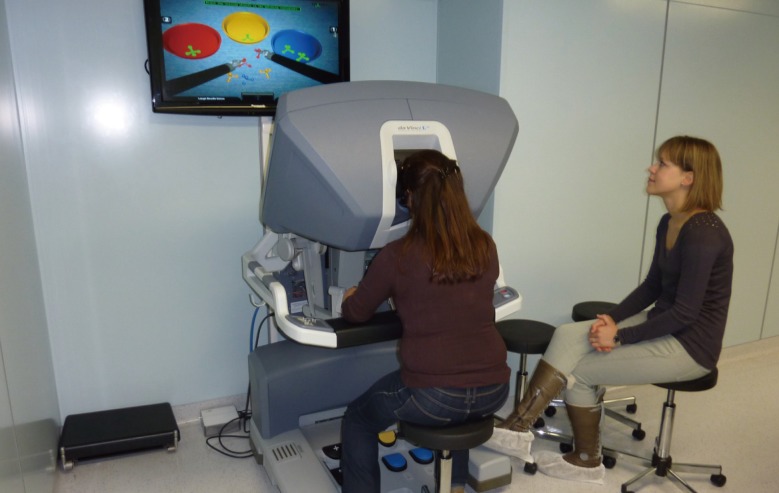
Simulator set-up
Installation of the da Vinci skills simulator for level 1 training. Several tasks of increasing difficulty can be developed, including vascular sutures. Precise measures of performance allow for self-education.
The second level is to use telemicrosurgical sutures on inexpensive models that pose no ethical dilemma in terms of animal testing. We use calibrated earthworms (Lombricus rubellus) with an average length of 60 mm and a mean outer diameter of 3 mm (Fig. 4). Each worm is anaesthetized by soaking in a 1% xylocaine solution until a significant slowdown in locomotor activity is observed. Each end of the worm is then cut with a scalpel followed by gentle finger pressure applied from one end to the other in order to completely eviscerate the worm. One obtains a hollow tube, the lumen, which replicates the lumen of a vessel of an average internal diameter of 2 mm. A frank cut in the middle of the model produces two segments of equal length ready to be anastomosed [12]. After anastomosis, the permeability and tightness of the model can be tested by injecting saline solution through one end [13]. It is also possible to use the femoral vessels of chicken thighs (Fig. 5). However, this model is less attractive than the worm because of its higher cost and the greater difficulty of its preparation.
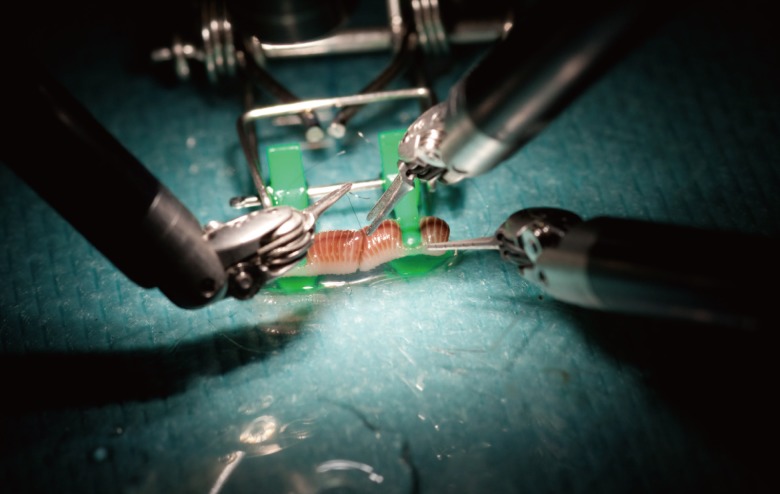
Earthworm model
Preparation of one worm for level 2 training. A terminoterminal telemicrosurgical anatomosis is realized with a 10/0 nylon thread.
Chicken artery model
Preparation of a femoral artery of a chicken leg for level 2 training. A terminoterminal telemicrosurgical anatomosis is realized with a 10/0 nylon thread.
The third level is to perform telemicrosurgical sutures on living models. The rat model for instance, must be used in accordance with current legislation on animal experimentation. The telemicrosurgeon, as well as the microsurgeon, must learn to perfectly master the vascular sutures: venous and arterial (Fig. 6) [14] and the nerve suture techniques (Fig. 7) [15].
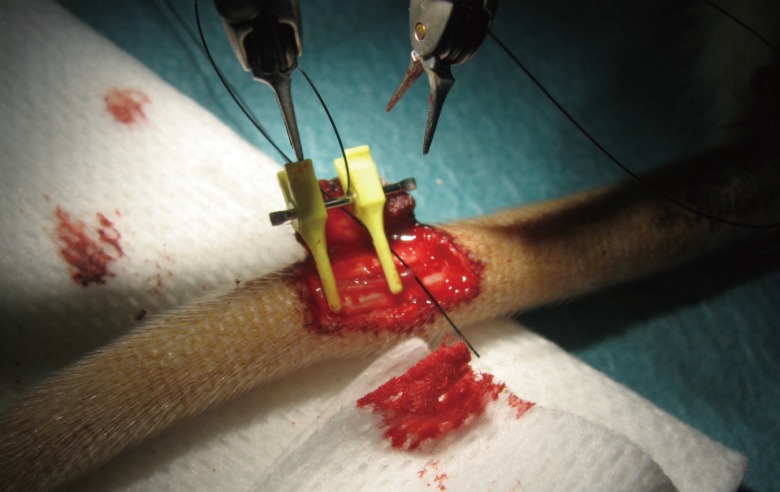
Rat artery model
Preparation of an artery of the tail of a rat for level 3 training. A terminoterminal telemicrosurgical anatomosis is realized with a 10/0 nylon thread.
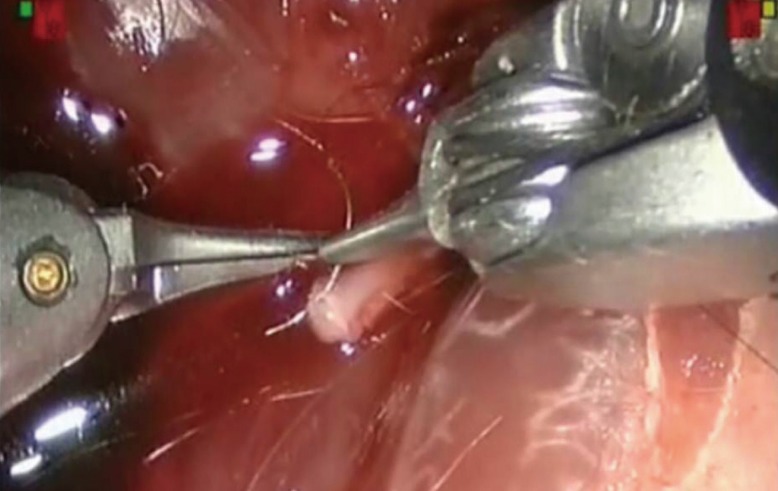
Rat nerve model
Preparation of a rat sciatic nerve for level 3 training. A terminoterminal telemicrosurgical anatomosis is realized with a 10/0 nylon thread.
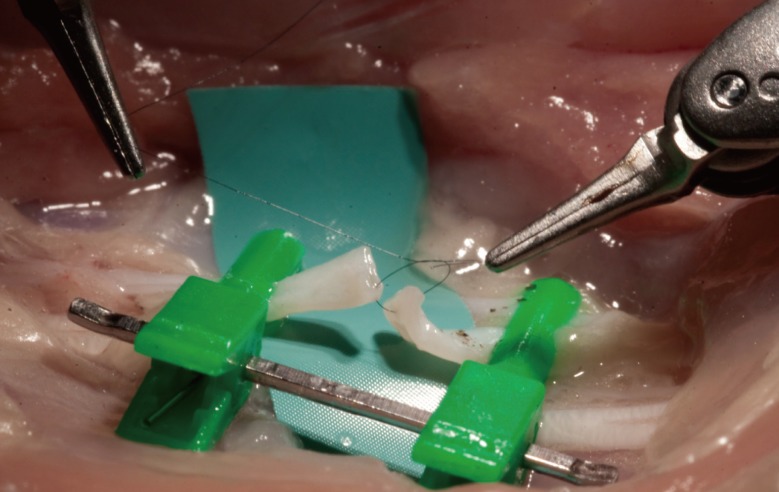
The fourth level is to perform more complex telemicrosurgical maneuvers. A thawed fresh human cadaver is an ideal model on which to learn to manipulate nerve regrowth scaffolds (Fig. 8), and to make pedicled flaps (Fig. 9) [16] and free flaps [17]. Reimplantations on living animals can also be performed [18].
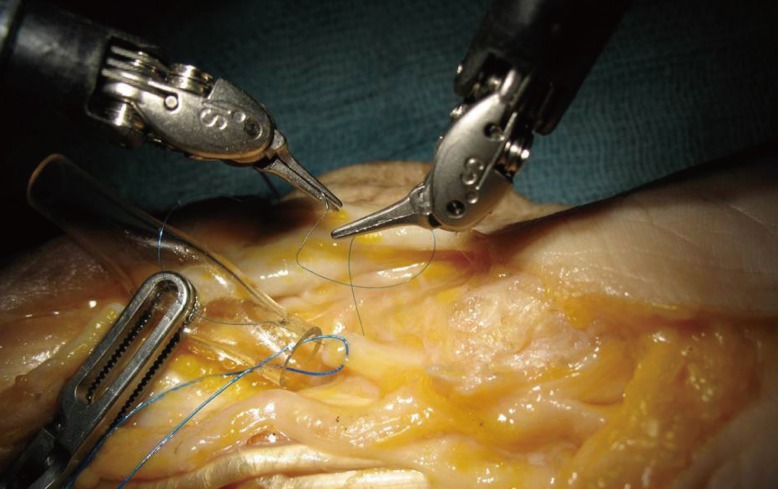
Cadaver nerve model
Preparation of a fresh human cadaver forearm for level 4 training. A graft of a nerve median is realized with a nerve guide with a 7/0 nylon thread.
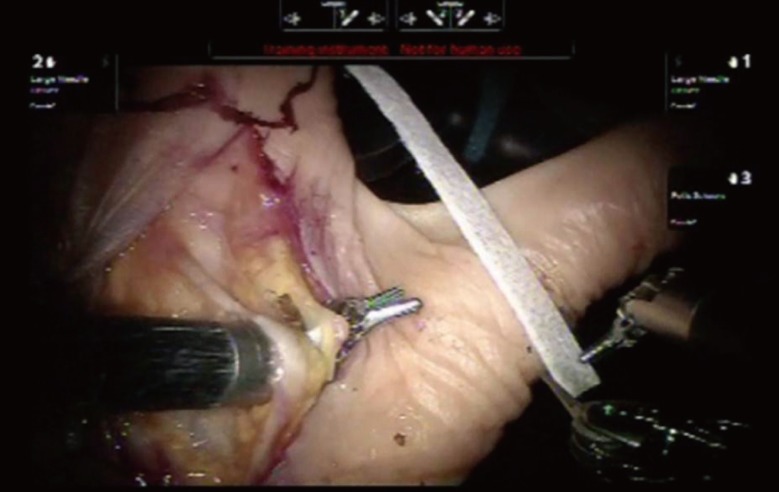
Cadaver flap model
Preparation of a fresh human cadaver hand for level 4 training. The pedicle of the first dorsal interosseous space is isolated before raising a kite flap.
The fifth and final level is reserved for endoscopic telemicrosurgery. It consists of telemicrosurgical manuevers through minimally invasive incisions. Reconstructive surgery of the brachial plexus [19] or the removal of large flaps of the latissimus dorsi muscle or of the rectus abdominis muscle [20] are particularly suited to endoscopic telemicrosurgery.
METHODS OF ASSESSMENT
Despite advances in surgical training, microsurgical training is still based on an apprenticeship model. Considering the complexity of microsurgery and the consequences of failure, the lack of a standardized, quantitative system to evaluate surgeons' skill, provide feedback, and measure training endpoints raises major quality control issues.
The Structured Assessment of Microsurgical Skills (SAMS) is a model designed to assess technical skills during microsurgery that was formulated from other assessment tools such as the Imperial College Surgical Assessment Device (ICSAD) and the Observed Structured Assessment of Technical Skills (OSATS) [21-24]. In a recent study, we validated the SAMS instrument and applied it to a cohort of microsurgical trainees, demonstrating acceptable inter-rater reliability and a reproducible learning curve [25].
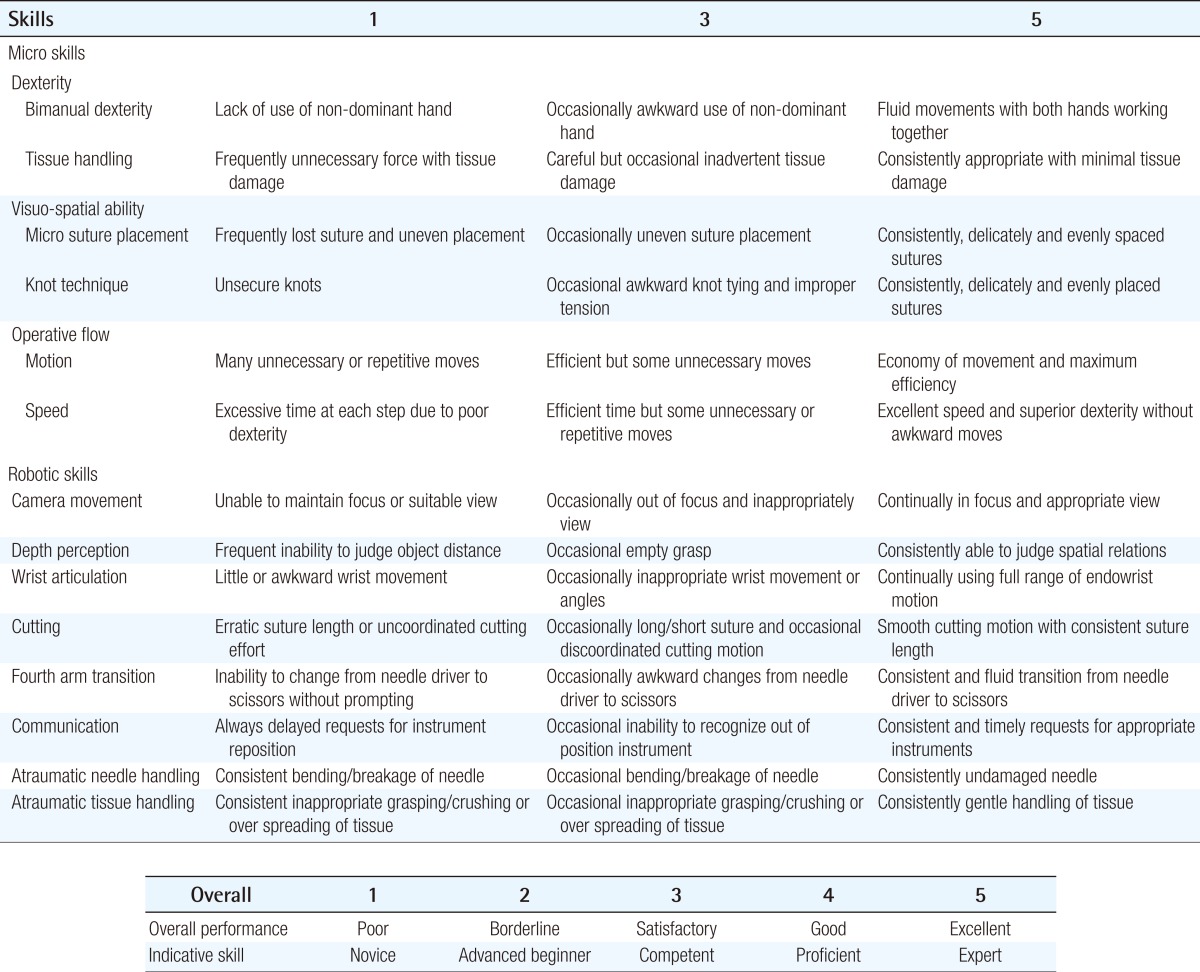
Robotic microsurgical skill combines many of the same principles and specific skill techniques as conventional microsurgery, but has the additional skill set that is unique to the use of the surgical robot. In order to capture this blend of skills for robotic microsurgery, we combined a validated robotic surgical rating system [26,27] with the SAMS, to create the Structured Assessment of Robotic Microsurgical Skill (SARMS) (Table 1). This evaluation system combines two separately validated skills assessment systems into a single evaluation system that encompasses both robotic skills and microsurgical skills. The SARMS is in the process of being validated by testing inter- and intra-rater reliability in a laboratory model. This is the first evaluation system of its kind and will serve as a guide to robotic microsurgical credentialing, as well as training endpoints for a robotic microsurgical curriculum. This will also serve as a research tool to help determine the learning curve for robotic microsurgical skills.
In addition to the SARMS, a demographic survey (Table 2) will be distributed in order to determine if years of training, level of training, gender, handedness, or previous experience with robotic, microsurgical, or laparascopic training, or video games has any effect on robotic microsurgical skill acquisition.
ORGANIZATION OF BASIC COURSE SKILL
Since 2009, RAMSES has organized foundation telemicrosurgery courses, twice a year, at the IRCAD facility, Strasbourg, Europe. A basic course program will open soon in Florida, USA. Each course welcomes a maximum of 6 participants from different specialties: urology, plastic surgery, hand surgery, orthopedics, ophthalmology, and neurosurgery. Up to the present, 42 surgeons from 11 countries have attended the basic courses (Japan, Singapore, Brazil, USA, Belgium, France, Netherlands, Spain, Switzerland, Turkey, and Kuwait). Two to three expert members of RAMSES supervise the participants. Each class lasts a full day. In the morning, seminars are given by engineers of the Intuitive Surgical Company and by RAMSES experts. The program includes the history of medical robotics, description of the robots, presentation of the telemicrosurgical models, and current clinical applications. The morning session ends at the laboratory, with a demonstration of the setup of the da Vinci robot by an engineer from the company Intuitive Surgical. The afternoon is devoted to a practical workshop. For a configuration of six participants, participants are divided into 3 groups of 2 people; 1 group of level 1 on the simulator and 2 groups of level 2 or 3 on the robot. The first group goes to the console of a da Vinci skills simulator to perform exercises at level 1. The 2 other groups work with each of the two da Vinci SI robots, each equipped with 2 consoles: 1 main operator console and 1 assistant console. Level 2 and 3 exercises can be done during the basic course. A certificate is issued to each participant who has completed the training.
Advanced courses on freshly thawed cadavers are planned on demand to acquire levels 4 and 5. The type of procedures performed depends on the participant's specialty according to their desire to develop a particular surgical technique.
In conclusion, the development of a basic course in telemicrosurgery or robotically assisted microsurgery is essential to ensure the credibility of this new discipline, to train future users, and develop new interventions.
Notes
Supported by RAMSES: The Robotic Assisted Microsurgical and Endoscopic Society (http://www.roboticmicrosurgeons.org/publications/).
No potential conflict of interest relevant to this article was reported.