Multiple Sparganosis in an Immunosuppressed Patient
Article information
Most of the parasitic infections that occur in Far East Asia including China, Japan, and Korea result from ingestion of infected water or the raw flesh of snakes or frogs.
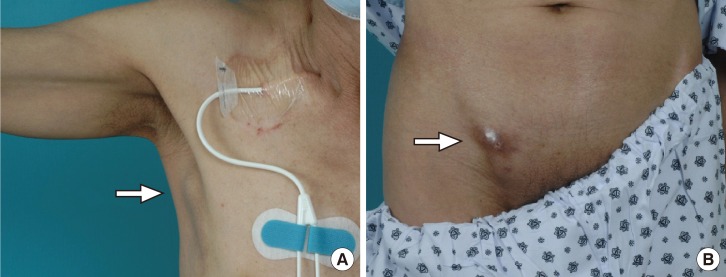
The most common clinical manifestation of sparganosis is a subcutaneous mass or lump in the breast, abdominal wall, scrotum, lower extremity, or chest wall. The larvae can invade tissues including muscle, intestine, eye, brain, and spinal cord and can also cause pericardial effusion. The mass may be mistaken for a malignant tumor, thereby causing difficulty in terms of diagnosis and treatment. In this article, we report an interesting case of sparganosis that presented as two separate subcutaneous masses involving the axillary and inguinal area in an immunosuppressed patient 45 years after the initial ingestion of contaminated food. A 61-year-old man visited our clinic with two 3 cm painless, movable, hard masses in the right axilla and inguinal area (Fig. 1). He had a medical history of acute lymphoma and had received an allo-peripheral stem cell transplant (PBSCT) 1 week prior to visiting our clinic. Immunosuppressive therapy was maintained with cyclosporine after PBSCT. The masses in the inguinal and axillary regions were suddenly observed following PBSCT, and the size of the masses increased during the first week. Routine laboratory tests were unremarkable and no eosinophilia was noted. Computerized tomography showed two 5 cm×3 cm×2 cm round nodules located in the subcutaneous layer (Fig. 2). Recent results from a punch biopsy from the inguinal area revealed sparganosis and suggested the potential for axillary lymphadenopathy, which was also diagnosed by computerized tomography as sparganosis. The patient subsequently recalled that he had eaten the raw flesh of a snake about 45 years earlier, during his childhood.
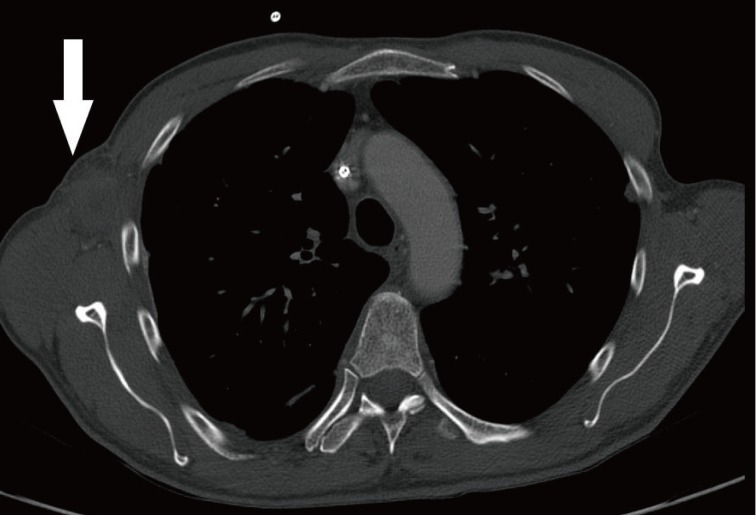
Chest contrast computed tomography findings (white arrow). 5 cm×3 cm×2 cm mass indicative of sparganosis in the right axillary area.
During the operation, small elliptical incisions were made on the upper portion of each mass. White and shiny masses were observed through the incision and the well-demarcated hard masses were completely removed along with hard palpable lymph nodes around the masses. The histological examination confirmed sparganosis, showing degenerative parasitic organisms with chronic inflammation consisting of numerous eosinophils (Fig. 3). Spargana larvae were found within the masses and granulation tissue with endothelial enlargement was also observed (Fig. 4). No antihelminthic drug was used, and the postoperative course was uneventful. One year postoperatively, the patient had no symptoms and showed no signs of recurrence of any lesion. Sparganosis caused by the migrating procercoid larvae of Diphyllobothrium mansoni is an extremely rare disease. The most common route of infection is via ingestion of contaminated water containing procercoids, which can penetrate into the intestine and migrate to the muscle or subcutaneous tissue [1]. The second route is from ingestion of raw or partially cooked fish, frogs, snakes, and chickens. Infection in this case also occurs from migration of the procercoid through the intestine. The third route of infection may occur from poultice application that may be infested with Cyclops containing procercoids that are utilized to open wounds or eyes.
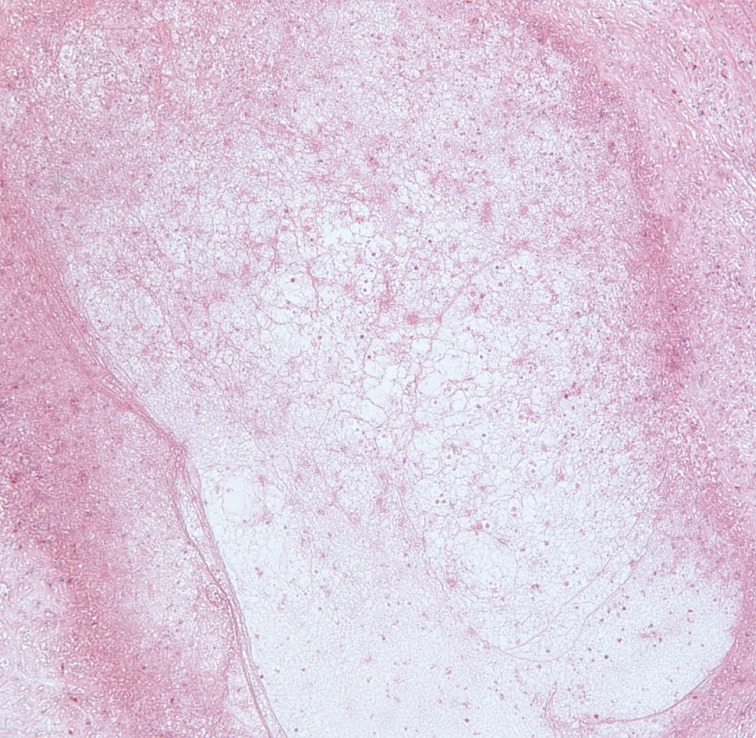
Histologic findings of the right inguinal area. Degenerated larvae with chronic inflammation are shown (H&E, ×40).
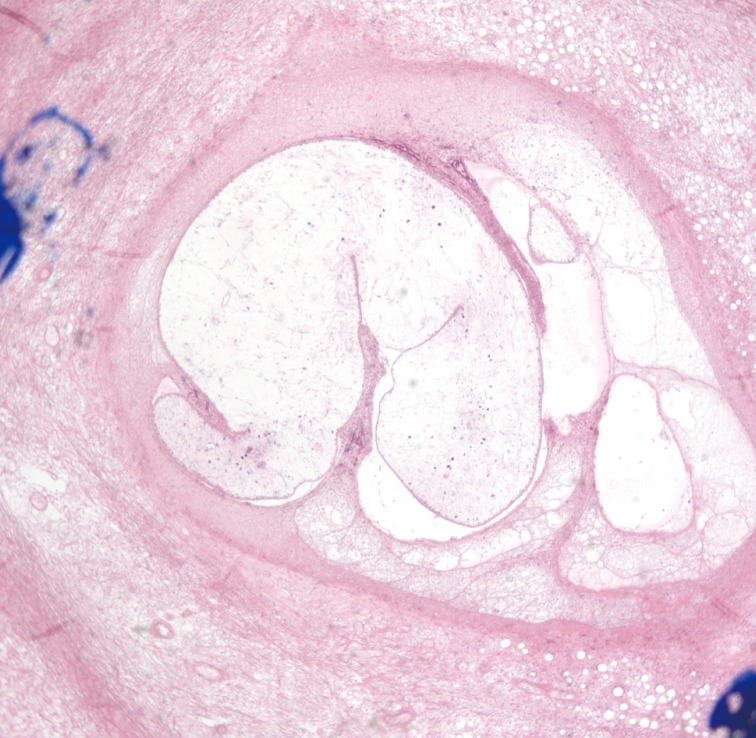
Histologic findings of the right axillary area. Degenerated larvae with chronic inflammation are shown (H&E, ×40).
Ingestion of the raw flesh of frogs or snakes is the most important infection route in East Asia. Interestingly, Korea experiences more frequent occurrences of sparagnosis than any other Asian country.
Sparganosis manifests as a migrating subcutaneous nodule in the abdominal wall, breast, lower extremity, or scrotum [2-4]. The ingested spargana can invade various organs, such as the eye, subcutaneous tissues, abdominal wall, brain, spinal cord, lung, or breast [1-3]. In the case of the genitourinary system, the involvement of the epididymis, spermatic cord, penis, retroperitoneum, and ureter have been reported [2-4]. Patients with sparganosis present clinically with vague or indeterminate symptoms. Importantly, distinguishing sparganosis from malignancy remains a significant concern.
While the rate of sparganosis has decreased with economic development and advancements in sanitation, surgeons still encounter patients with sparganosis in the clinical setting. Furthermore, the most frequent infection route is also changing. Therefore, a careful history is required in order to diagnose sparganosis. A definite diagnosis of sparganosis can be performed by excisional biopsy. Utilizing magnetic resonance imaging or computed tomography reveals characteristic signs of sparganosis: a band-like structure with a coiled hypo-echoic body. However, these imaging studies provide limited evidence for making a diagnosis. In a recent article, screening using enzyme-linked immunosorbent assay from the serum was reported as a reasonable method for diagnosis with high sensitivity and specificity.
Sparganum larvae can penetrate anywhere in the body, where they can migrate anywhere, including subcutaneous tissue. When the sparganum is ingested, it penetrates the intestinal wall to reach the peritoneal cavity and begins to migrate systemically. The longevity of spargana is thought to be under 1 year. However, some articles have reported finding a live worm after more than 10 years. Kubota and Itoh [4] reported a case of orbital sparganosis with the patient suffering from a painful nodule after 10 years and Koo et al. [2] also reported a case that mimicked a varicose vein after 10 years. We experienced an interesting case of sparganosis that had been latent for 45 years without symptoms but manifested with subcutaneous nodules after immunosuppressive therapy. In this case, we concluded that the larvae were dead in the lymph nodes and the immunosuppressant therapy might have caused lymphadenopathy. Although the exact length of latency is not specific in most published articles, our case could be the longest known latent period of spargnosis. The long-term survival of the parasites could result from their immunomodulatory ability with respect to the host. Further research is required in order to understand the role of sparganum as a valuable model for alleviating symptoms of immune-mediated disease.
Human sparganosis is an extremely rare disease, even in endemic countries, and the clinical presentations are diverse, including non-specific pain, vague discomfort, palpable masses, headache, or symptoms related to the involved organs [5]. The infection route, latent period, and symptoms of the disease are changing with economic development. Therefore, surgeons should be aware of the clinical characteristics in order to make a diagnosis. In this respect, a careful patient history is most important. In our case, we speculate that the longevity of spargana could have been up to 45 years with a parasitic infection occurring in two sites following a change in the patient's immune status.
Notes
No potential conflict of interest relevant to this article was reported.