Application of Local Axial Flaps to Scalp Reconstruction
Article information
Abstract
Background
Scalp defects may be caused by various etiological factors, and they represent a significant surgical and aesthetic concern. Various surgical techniques can be applied for reconstructive work such as primary closure, skin grafting, pedicled or free flaps. In this article, the authors share their clinical experience with scalp operations using the technique of local flaps and discuss the application of this method from the perspective of not only the size of the defect, but also in relation to the anatomical area, quality of surrounding tissue, and patient's condition.
Methods
During the period from December 2007 to December 2012, 13 patients with various scalp defects, aged 11 to 86 years, underwent reconstruction with local pedicle flaps. The indications were based on the patients' condition (age, sex, quality of surrounding tissue, and comorbidities) and wound parameters. Depending on the size of the defects, they were classified into three groups as follows: large, 20 to 50 cm2; very large, 50 to 100 cm2; extremely large, 100 cm2. The location was defined as peripheral (frontal, temporal, occipital), central, or combined (more than one area). We performed reconstruction with 11 single transposition flaps and 1 bipedicle with a skin graft on the donor area, and 2 advancement flaps in 1 patient.
Results
In all of the patients, complete tissue coverage was achieved. The recovery was relatively quick, without hematoma, seroma, or infections. The flaps survived entirely.
Conclusions
Local flaps are widely used in scalp reconstruction since they provide healthy, stable, hair-bearing tissue and require a short healing time for the patients.
INTRODUCTION
Scalp defects may be caused by various etiological factors and present a significant surgical and aesthetic concern. Different surgical techniques can be applied for reconstructive work such as primary closure with adjacent tissue, skin grafting, and random, pedicled (local or regional), or free flaps [1]. Due to the limited mobility of the scalp tissue, only small defects can be closed primarily. Skin grafting is an appropriate option in cases when the pericranium is present. However, in cases of large scalp defects with denuded periosteum calvarium, exposed dura, or cerebrospinal fluid leaks, neither primary closure nor skin grafting are applicable. Such injuries require local, distant vascularized flaps or free tissue transfer. In most cases, local flaps are the first method of choice.
In this article, the authors share their clinical experience with scalp reconstruction using the technique of local flaps and discuss the application of this method in view of not only the size of the defect, but also in relation to the anatomical area and patient's condition.
METHODS
During the period from December 2007 to December 2012, 13 patients with scalp defects underwent reconstruction with local flaps. The wound etiology varied: deep burns (n=5); basal cell carcinoma (n=5); a horse bite wound (n=1), and postoperative complications after tumor removal (n=2). The indications for the application of local flaps were based on two factors: the patients' condition and wound parameters. We analyzed parameters such as age, sex, quality of surrounding tissue, and comorbidities. The mean age was 61.7 years (ranging from 11 to 86 years). There were 11 men and 2 women. The surrounding tissues were categorized as good and satisfactory (when part of it was had cicatrices or superficial burns). Accompanying comorbidities were taken into account, but were not regarded as contraindications. The final choice of the reconstructive method was determined with regard to wound related parameters: size, location and depth of the defect. The defect size was classified into three groups as follows: large, 20 to 50 cm2; very large, 50 to 100 cm2; and extremely large, 100 cm2. The location was regarded as peripheral (frontal, temporal, or occipital), central, or combined (more than one area). The depth of the wound was determined to be skin and galea loss; missing pericranium; or bone defect.
The type of flaps used in this patient group were as follows: 1) A single large transposition flap was used in 11 patients with a defect size of 20 to 120 cm2 and a peripheral or combined (central and peripheral) location. The surrounding tissue was soft and pliable in 8 patients and satisfactory in 4 cases. 2) An advancement flap was used in 1 patient with a centrally located wound (40 cm2), skin and galea loss only, and elastic surrounding tissue. 3) A bipedicle flap was used in 1 case with defects located in three areas (frontalis, parietal, occipital) and a size of 391 cm2. There was damage to the skin, galea, and pericranium, and the surrounding tissue was of good quality.
All of the flaps were planned to be two to three times larger than the measurements of the defect in order to achieve wound coverage without tension. In the case of the bone defect, the calvarium was reconstructed with a prosthetic plate and then covered with a local flap.
The vascular pedicle was based on the right or left occipital artery in 8 of the patients, in 4 cases the superficial temporal and retroauricular arteries were included, and in one patient, the flap supply was provided by the left occipital, right supraorbital and supratrochlear arteries. The flaps were elevated by extensive undermining in the areolar tissues between the galea and the pericranium. During the dissection, care was taken to avoid injury to the periosteum of the calvaria. Whenever there was needw of additional tissue gain and mobility of the flap, we scored the galea at 1-cm intervals in the direction of the flap movement. The donor site was covered with a split-thickness skin graft in 12 of the cases, and in one patient the wound was closed primarily. Vacuum drains and compressive bandages were applied in all patients for 48 hours.
RESULTS
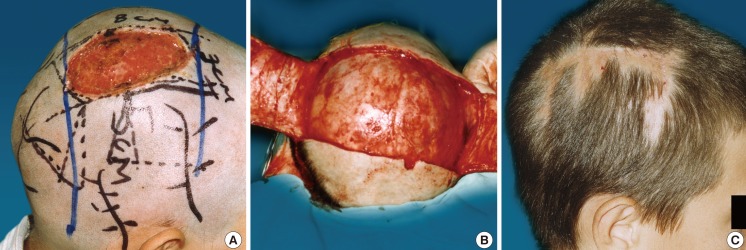
In the early and late postoperative period, the qualities of coverage and recipient and donor morbidities were followed up. In all cases, complete tissue coverage has been achieved. All of the flaps healed and survived completely. In three of the patients, a partial skin graft lesion was observed-in two of them due to an injured periosteum during flap dissection. The lamina externa was perforated and after the growth of granulation tissue, a skin graft was applied. Dehiscence of the surgical suture at 2 cm was observed in one patient. After the excision of the wound edges, secondary suturing was done. Only two of the patients requested the removal of the cone that formed at the base of the flap. Table 1 displays the results from the surgical work and the flaps that were applied according to the patient's condition and defect characteristics (Figs. 1-4).
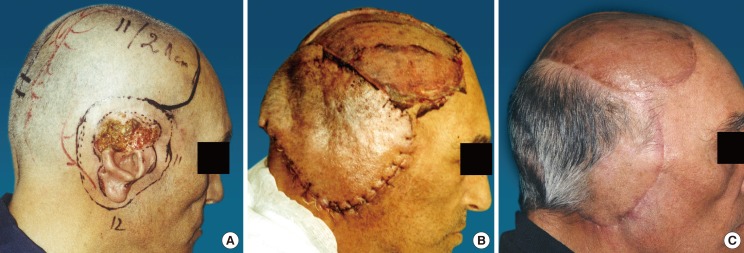
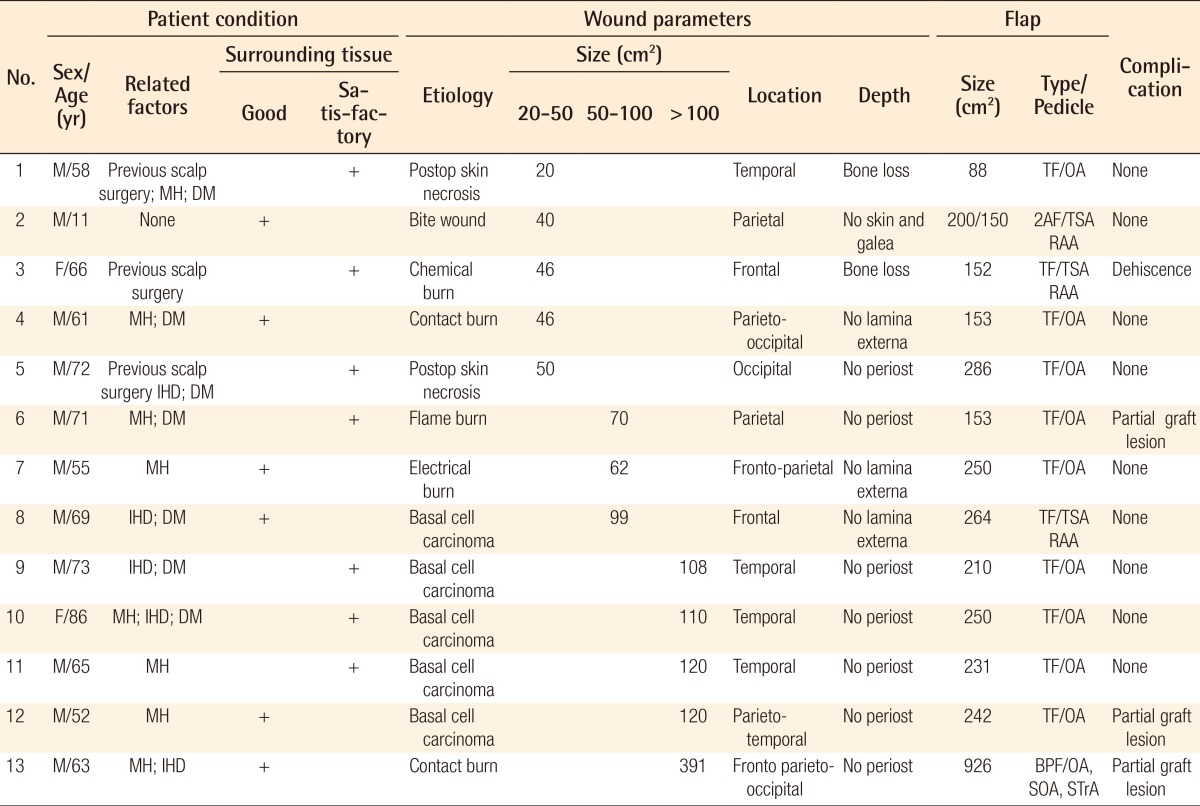
Case 1 (patient 11) preoperative and postoperative images
(A) Basal cell carcinoma in the right temporoauricular area, which comprised the upper 2/3 of the ear. The size of the defect was 120 cm2. (B) Early results: 5 days after coverage of the defect with a flap measuring 231 cm2, based on the right occipital artery. The donor area was covered with a skin graft. (C) Both the flap and the skin graft survived completely. Postoperative results: 30th postoperative day.
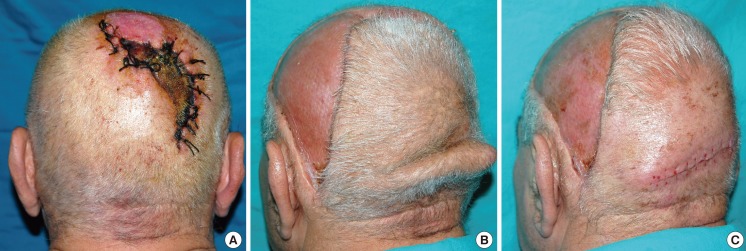
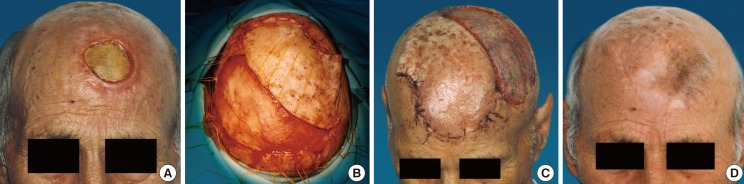
Case 4 (patient 5) preoperative and postoperative images
(A) A patient who had undergone tumor removal in whom the wound had been closed under tension, causing subsequent necrosis of the skin 50 cm2 in size. (B) The defect was covered with a large flap measuring 286 cm2, based on the left occipital artery. (C) After the excision of the cone: 30 days postoperatively.
DISCUSSION
Reconstructive surgery of the scalp following oncological resection, various injuries, deep burns, or postoperative complications such as tissue necrosis can be quite demanding. Perfect reconstruction may only be achieved by using tissues with the same thickness and hair growth, which allows for adequate coverage, mobility, and stability [2]. It is known that primary direct closure is usually the first method of choice when treating small (<5 cm2) wounds [3,4]. Skin grafting is a suitable technique when the wound bed is well-vascularized, but unsatisfactory functional and aesthetic results limit the application of this method [5,6]. When the defect is medium (5-20 cm2) or large in size (>20 cm2), with a denuded periosteum calvarium, exposed dura, or cerebrospinal fluid leaks, the correct reconstructive technique is the application of local, regional, or free flaps [4]. Local flaps provide the same tissue type as the scalp and, therefore, adequate coverage of defects [7,8]. They belong to the fasciocutaneous flaps and can be planned in various shapes depending on the size, localization, and depth of the wound. Each one of the five large arteries of the head can be the pedicle vessel [4,9,10]. The rich vascular network and the multitude of anastomosis allows for flexibility in the formation of the pedicle, as well as the form and the size of the flap [11].
Adequate reconstruction of medium-sized wounds can be achieved with local flaps followed by primary closure of the donor site [8]. This technique provides healthy, durable, and hair-bearing tissue, but the application of the method requires good quality of the surrounding tissue. In certain situations, galeal scoring may be performed to gain flap length and reduce tension. Care should be taken to perform scoring parallel to the blood vessels and avoid scoring too deeply. In cases of very large defects, local flaps large enough to cover the recipient area should be used, but for the donor site, a skin graft must be placed [12]. When applying this surgical approach, special attention should be paid to the area of the hairline and sideburns, which have to be preserved as much as possible. The donor site should be selected in the least aesthetically sensitive area depending on the defect [13].
Whenever we decide on the type of flap, we consider not only the size, but also the position of the defect. Advancement flaps or multiple flaps with primary closure may be applied in patients with elastic scalp tissue, a moderately sized defect, and a location in the central or parietal area of the scalp [14]. When the wound is peripheral or more than one zone is involved, the flaps must be both transposition flaps and larger in size. In addition, primary closure of the donor site is not feasible. We have applied advancement flaps in only one of the patients, whose defect was 40 cm2 and located in the parietal area. In all of the other cases, large transposition flaps with skin grafting for the donor area were used. Taking into account the limited elasticity of the scalp, flaps must be planned to be 2 to 3 times larger in size than the measurements of the wound. They should be elevated by dissection at the subgaleal plane and care must taken to avoid injuries to the pericranium. Apart from the size and position of the defect, another important factor when considering options for scalp reconstruction is wound depth [12]. Extensive lesions of the scalp involving not only the periosteum, but also the bone and dura require placing a bone transplant or titanium mesh, which are covered with the flap [15].
However, in extensively large defects, skin damaged by radiation treatment, a substantial bone loss, or when the defect covers more than 50 percent of the scalp, local tissues may not be the appropriate approach, and the alternatives are tissue expansion, distal pedicled or free flaps [15]. Tissue expansion is an effective method for enlarging tissues and achieving adequate coverage with local flaps, and in most cases the procedure has satisfactory functional and aesthetic outcomes [16]. However, unfortunately, this method was not suitable for immediate coverage in our patients for many reasons: a number of the defects were related to basal cell carcinoma and some of patients had only satisfactory skin pliability due to previous surgical scars. In addition, expansion is performed in several stages and requires plenty of time for obtaining optimal results and is not free of possible complications [17]. We consider tissue expanders to be a secondary treatment for achieving aesthetic improvement and they are applied only at a patient's request.
Planning and elevation of distal pedicled flaps demand long surgical hours and require extended recovery time for the patients [18]. These flaps have a relatively large size but a restricted range of mobilization, which limits their extension to the lateral or occipital areas of the scalp, and several surgical interventions are needed to model and adapt them to the delicate structures of the head. The comorbidities of our patients did not allow the use of these flaps due to the potentially high risk of ischemia of the flap. Furthermore, if a trained microsurgical team is not available for free tissue transfer, distal pedicled flaps are the feasible option for covering large composite defects [5].
Free flap transfer is a safe and reliable technique for covering large and deep calvarial defects [19-21]. These flaps are not hair bearing, but provide a good quantity of tissue with quality elasticity.
The method is preferred in case of bone infection and deficiency of local tissue [3].
Compared to regional and free flaps, treatment with local flaps is a safe, relatively short, and simple procedure unlikely to cause any major complications or demand special postoperative care. The cone that is formed at the base of the transposition flap may be surgically removed after one month upon request. Postoperative alopecia, when the donor site is covered with skin, may be corrected by expanders if the patient wishes.
In our view, local flaps are a sufficiently good technique for scalp reconstruction when the defects are large, with denuded periosteum calvaria, or with bone loss. Our results from the application of 14 local axial flaps indicate that complications were quite rare and did not affect the survival of the flaps.
Notes
No potential conflict of interest relevant to this article was reported.