Solitary Labial Metastasis of Adrenocortical Carcinoma Resembling a Cystic Tumor in a Child
Article information
Facial skin metastases account for less than 0.5% of patients with metastatic cancer and most of them originate from malignant melanoma [1]. Because the clinical presentations of skin metastasis are often nonspecific, early detection is very difficult. Adrenocortical carcinoma (ACC) is a rare tumor accounting for 0.2% of all childhood cancers and has an incidence of 1-2 cases per 1.7 million persons [2]. Until now, cutaneous metastases have rarely been reported. Herein, we report on a 4-year-old girl who presented with an elevated mass resembling a cystic tumor on her right upper lip that was diagnosed as metastatic ACC.
A 4-year-old girl who was diagnosed with Turner syndrome recently visited our hospital for the evaluation of a right maxillary area subcutaneous mass. The mass was 3.5 cm×2.5 cm in size and had developed 6 months earlier. It was round, non-tender and fixed and, clinically, seemed to be a cystic tumor.
The patient had a history of adrenalectomy due to left ACC 2 years earlier. At that time the tumor had been 7.5 cm×6.5 cm×4.5 cm in size and weighed 102 g (Fig. 1). The resection margins were tumorfree, and there was no lymph node enlargement or local invasion. According to the modified staging system of pediatric ACC, it was stage I. After complete resection of the ACC, no further symptoms or signs had developed until 18 months later, when she felt a palpable mass on her right maxillary area.
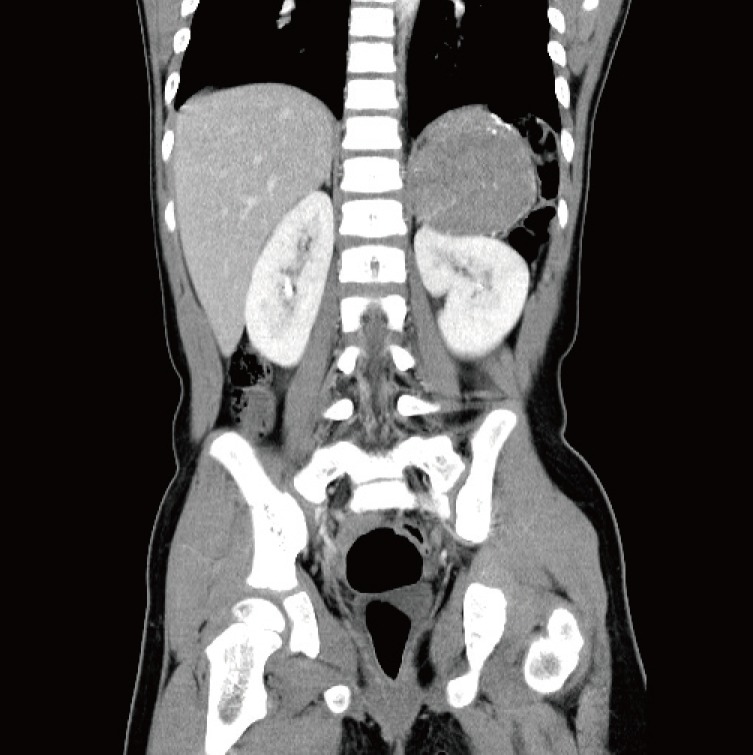
The patient had a history of adrenal cortical carcinoma 2 years before the metastatic presentation. There was a huge mass (7 cm×6 cm×4 cm) on the adrenal gland consistent with adrenal cortical carcinoma.
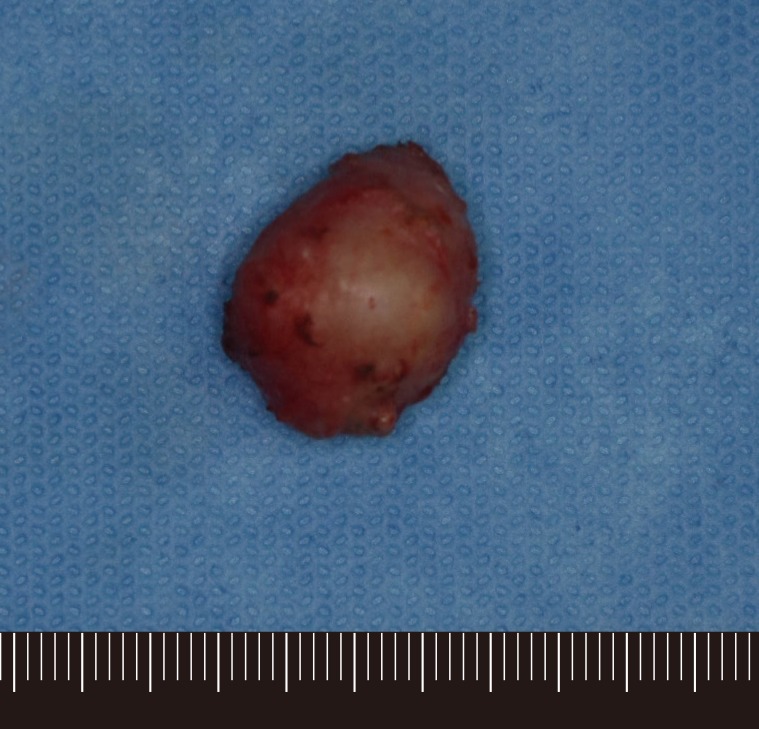
The preoperative computed tomography (CT) and ultrasonography of the maxillary mass indicated the possibility of a solid tumor (Fig. 2). The mass was deep in the subcutaneous level, so the approach was performed through the right upper oral mucosa. After penetrating the orbicularis oris muscle, a well-encapsulated mass was found (Fig. 3). The mass was overlying the subcutaneous and muscle layer. The mass was excised entirely, preserving normal adjacent tissue without any rupture. The specimen was 2.2 cm×1.5 cm×1.5 cm in size and sent to the pathology department (Fig. 4).
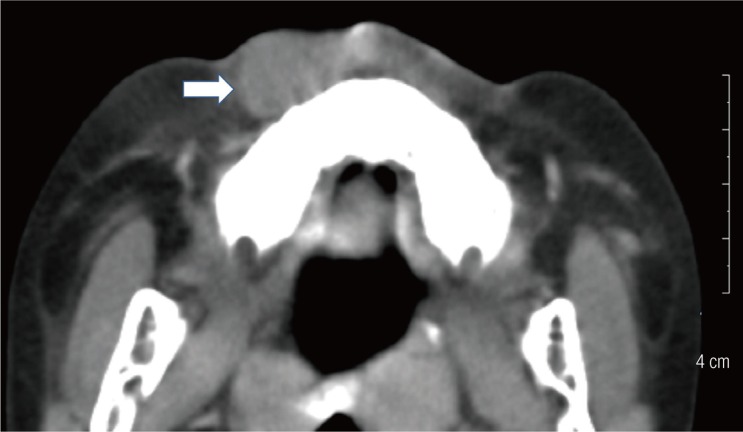
Preoperative computed tomographyfindings. Facial computed tomography findings showing a solid mass (white arrow) with irregular marginsin the right premaxillary area.
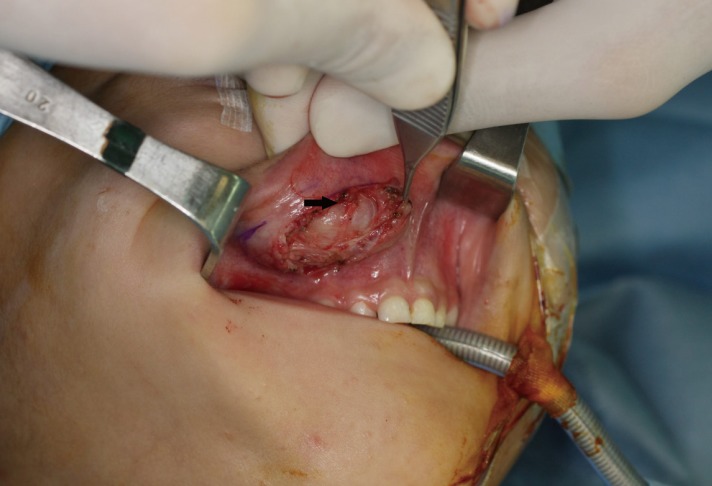
Intraoperative view. The well-encapsulated round mass (black arrow) was noted while the orbicular is oris muscle was being held with Adson tissue forceps.
Histological evaluation of the mass showed a high nuclear grade, atypical mitosis, sinusoidal invasion, and a mitotic rate of 40/50 in the high power field (Fig. 5). This histopathology was consistent with that of the ACC excised 2 years earlier. The laboratory studies of the hormone level revealed a normal range. Additionally, there was no evidence of lymph node enlargement or other organ metastasis in CT and positron emission tomography (PET)-CT evaluation. Therefore, she did not receive chemotherapy or radiation.
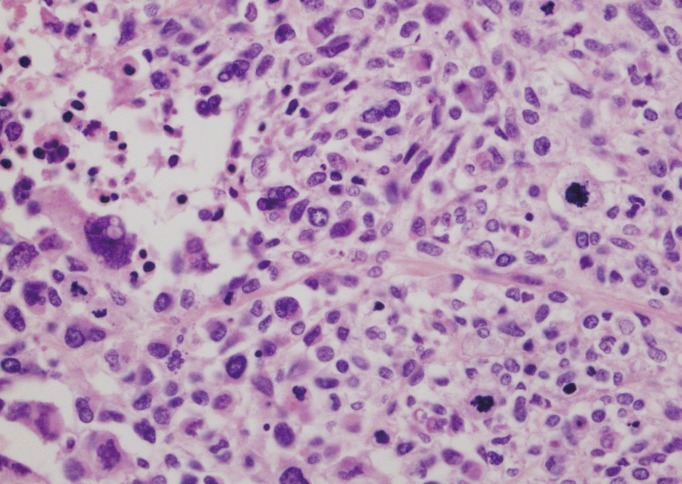
Pathologic findings. The high power view of the metastatic adrenocortical carcinoma shows pleomorphic tumor cells, increased mitoses, and necrosis similar to that of the previous adrenocortical carcinoma (H&E, × 400).
During 2 years of follow-up, there was no evidence of local recurrence and no distant metastasis in facial and abdominal CT or PET-CT evaluation.
ACC is a rare malignancy, and it is associated with p53 mutation in children. Generally, ACC can be classified as a functional or non-functional tumor. Children with ACC most commonly present with virilization. Although several studies have shown that patients with functional tumors have a slightly better outcome, other studies contradict this finding [3]. 75% of cases in children are localized to the adrenal gland (stage I and stage II); however, in adult patients, 30% to 85% of patients have distant metastasis at the time of presentation. The most common sites of metastasis are the liver, local lymph nodes, lung, peritoneum, and bone [4]. However, cutaneous metastases have rarely been reported. In the previous report, the facial metastatic lesion existed concomitantly with ACC, but in our case the metastatic lesion occurred 18 months after the excision of the primary ACC. Therefore, our case is the first report of ACC with delayed metastasis to the facial area.
For primary ACC, histopathologic findings of tumor necrosis, a mitotic rate of more than 5 of 50 in the high power field, and atypical mitotic figures are associated with reduced disease-free survival [4]. In metastatic ACC, poor prognostic factors are liver metastases, bone metastases, more than 5metastatic lesions, more than 2 tumoral organs, a high mitotic count of more than 20 of 50 in the high power field, and presence of atypical mitoses. In contrast, patients whose metastases were diagnosed more than 1 year after resection of the primary ACC had a better outcome than those who developed a recurrence before this date [5]. In our case, the poor prognostic factors were atypical mitosis and a high mitotic rate (40 of 50 in the high power field), which were found in both the original ACC 2 years earlier and the metastatic lesion. However, late recurrence of the metastasis (18 months after adrenalectomy) is a good prognostic factor.
In patients with advanced or metastatic tumors, resection of the metastatic disease is indicated if possible. Otherwise, they can be treated with adjuvant chemotherapy, mitotane, and radiation. There has been no analysis of the efficacy of adjuvant treatment in patients with advanced-stage disease [4]. If complete resection is possible, no further therapy is indicated. Close observation with imaging and endocrine studies is needed.
Our case is noteworthy because delayed metastasis of ACC to the facial skin is extremely rare, and there was only a solitary metastatic lesion without any tumor recurrences or distant metastases.
The clinical presentations of skin metastasis are highly variable, so they may go unnoticed for a long time. Most skin metastasis appears as rapidly growing solitary or multiple round or subcutaneous nodules, and the lesion is usually painless [3]. In our case, the mass had round, non-tender, and fixed features. Because the mass seemed clinically to be a cystic tumor and no symptoms or signs had developed until 18 months after resection of the primary ACC, early detection of the skin metastasis was difficult. Additionally, there was no evidence of metastasis in the regular oncology work-up. Although the punch biopsy revealed capillary proliferation, we used imaging studies (CT and ultrasonography) with a high index of suspicion and performed a surgical excision for accurate diagnosis.
In conclusion, careful examination with various diagnostic methods and total excisional biopsy are recommended for early detection of metastasis if there is any suspicious skin lesion in patients with a history of malignancy.
Notes
No potential conflict of interest relevant to this article was reported.