Regional Analysis of Soft Tissue Thickness on Korean Buttocks and Application to Fasciocutaneous Flap Design
Article information
Abstract
Background
Various shapes and designs of the gluteal artery perforator flap have been used for treating sacral pressure sores and reconstructing breasts. To establish the ideal fasciocutaneous flap design for use in the gluteal area, the soft tissue thickness distribution was measured.
Methods
Twenty-one buttocks of adult Korean cadavers were analyzed through rectangular subfascial dissection. Each buttock was divided horizontally into 10 sections and vertically into 10 sections, and then, the thickness at the corners of the sections was measured. For the sake of comparison and statistical verification with living bodies, computed tomography (CT) images of 120 buttocks of patients were randomly selected. Five horizontal sections and 4 vertical sections were made, and the thickness at each corner was recorded.
Results
According to the dissection and the CT images, the area with the thinnest soft tissues in the buttock was around the posterior superior iliac spine, close to the sacral area. The thickest area was the superolateral area of the buttock, which was 3.24 times and 2.15 times thicker than the thinnest area in the studies on cadaver anatomy and the CT images, respectively.
Conclusions
The thickness of the soft tissues in the buttocks differed by area. The superolateral area had the thickest soft tissues, and the superomedial area had the thinnest. This study includes information on the distribution of the thickness of the gluteal soft tissues of Koreans. The outcome of this study may contribute to the design of effective local flaps for pressure sore reconstruction and free flaps for breast reconstruction.
INTRODUCTION
The perforator flap, which was introduced in the 1980s, has been widely used in many reconstruction fields [1]. Since Koshima et al. [2] introduced sacral pressure sore reconstruction using a gluteal perforator flap from the sacral area perforator, the gluteal area has been frequently used as a donor site of perforator flaps because the area has a sufficient volume of soft tissues with thick fat and a considerable number of blood vessels that have many perforators. Reconstruction and anatomical studies using the perforators of the gluteal area have focused on the superior buttocks arterial perforator flap and the inferior buttocks arterial perforator flap [3,4]. In addition to the local flap, Fujino et al. [5] introduced a method of breast reconstruction in 1975 that uses the free flap obtained from gluteal soft tissues. When gluteal soft tissues are used for breast reconstruction, a sufficient volume for surgery is available, and the scar can be cosmetically hidden beneath the clothes. Accordingly, this method has been widely used when breast reconstruction using abdominal fat is not possible.
In flap design, the location and size of the defects and the blood supply are important factors to consider. The availability of a flap with the appropriate volume and shape is also important. The flaps obtained from the gluteal soft tissues are used in various methods. For the reconstruction of sacral pressure sores, flaps with various shapes, including rotation flaps, advancement flaps, and propeller-type flaps, are used [6,7,8]. In breast reconstruction, flaps with semilunar or oval shapes, which are obtained from the gluteal superior or inferior area, are used [9,10,11 12].
For the reconstruction of pressure sores, flaps that have a sufficient cushion with abundant blood flow and thick tissue are recommended. For breast reconstruction, a flap designed to have a thin superior area and a thick inferolateral area is desirable, considering the three-dimensional (3D) shape of the breast. To choose the location of the optimal donor site for reconstruction and the shape of the flap, not only the location of the gluteal perforator but also the thickness of the soft tissues must be determined. Many studies on the location of the gluteal perforator have been reported [3,13,14,15]. However, no study on the distribution of the 3D thickness of the gluteal soft tissues has been reported. If perforator locations and flap thickness are considered when choosing the donor site of fasciocutaneous flaps, the optimal flap design for reconstruction may be achieved. Therefore, the thickness distribution of the gluteal soft tissues was confirmed in this study.
METHODS
Anatomical study
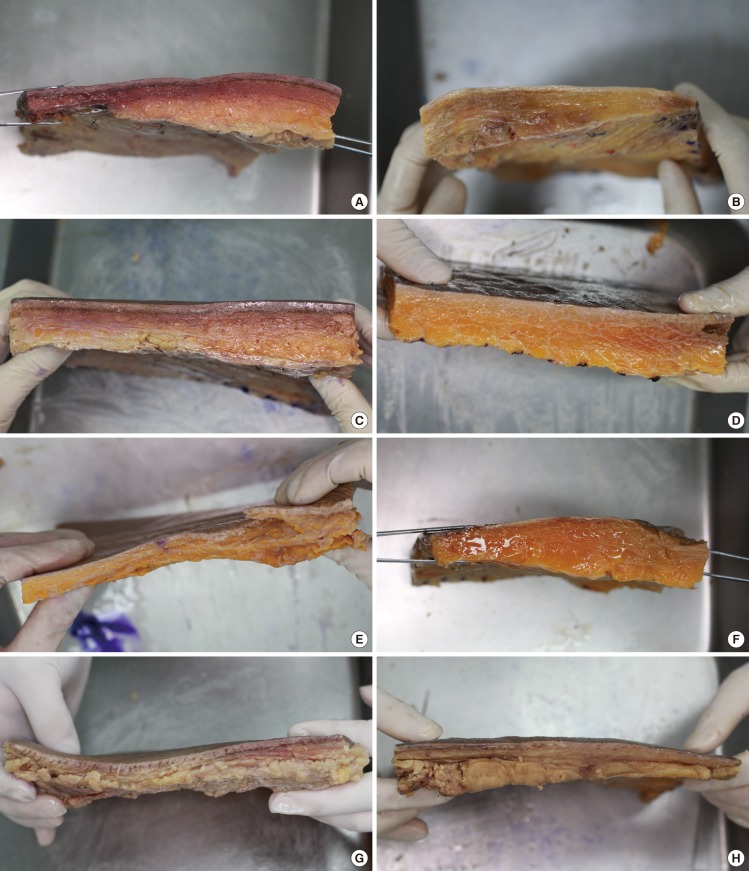
Twenty-one buttocks of 11 adult Korean cadavers were dissected. One buttock was severely damaged and was excluded. A rectangular flap was designed with the posterior superior iliac spine (PSIS) and the great trochanter (GT) as the superomedial and inferolateral boundaries, respectively. An incision was made at the lateral side of the skin, and a rectangular flap was lifted through subfascial dissection. The separated flap maintained a curved surface. The flap was fixed for 10 days with a typical fixative in a container with a flat bottom. Then, the flattened flap was horizontally divided into 11 sections (marked A to K from the medial side to the lateral side) and vertically divided into 11 sections (marked 0 to 10 from the superior area to the inferior area). Next, the flap was dissected to measure the thickness of the corner of each segment, including that of the tissues of the fascia and the skin (Fig. 1).
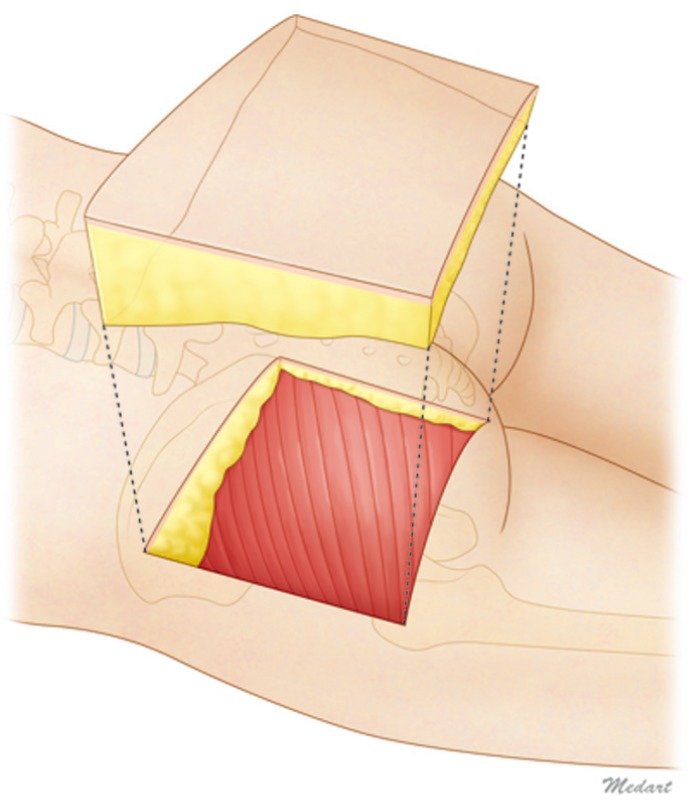
Schematic representation of the cadaver study
The specimen's superomedial margin was the posterior superior iliac spine, and the inferolateral margin was the great trochanter. The rectangular fasciocutaneous flap was elevated. Each flap was divided into ten areas horizontally and ten areas vertically, and the thickness of each corner was measured.
Biotrepy through CT Images
Data collection
For comparison with living bodies and statistical verification, the computed tomography (CT) images of 60 patients aged 31 to 60 years were analyzed. Among the patients who underwent an abdominal and pelvic CT scans, 10 male and 10 female patients were randomly selected from each 10-year age category. The age, height, weight, and body mass index (BMI) of the patients were recorded. A total of 120 CT images of the buttocks were obtained, and the distance between the PSIS and the GT was divided into 5 sections mediolaterally (marked A to E from the medial to the lateral sides) and 4 sections superoinferiorly (marked 1 to 4 from the superior to the inferior areas). Then, the vertical distance from the fascia to the skin at the corner of each section was measured and recorded.
Statistical analysis
SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Considering the individual differences in the thickness of the soft tissues, the thickness index (TI), which was calculated by dividing the thickness of each section by the thickness of the corner of the superomedial segment, was used for the statistical analysis. Further, a one-way analysis of variance (ANOVA) test was conducted using the measurements of each area observed in the CT images, in order to confirm the significant difference in the TIs in each area. The effects of age, height, weight, and BMI on the TI of the thickest area were analyzed through a correlation analysis. A statistically significant difference was defined as that with a P-value of less than 0.05.
RESULTS
Anatomical study
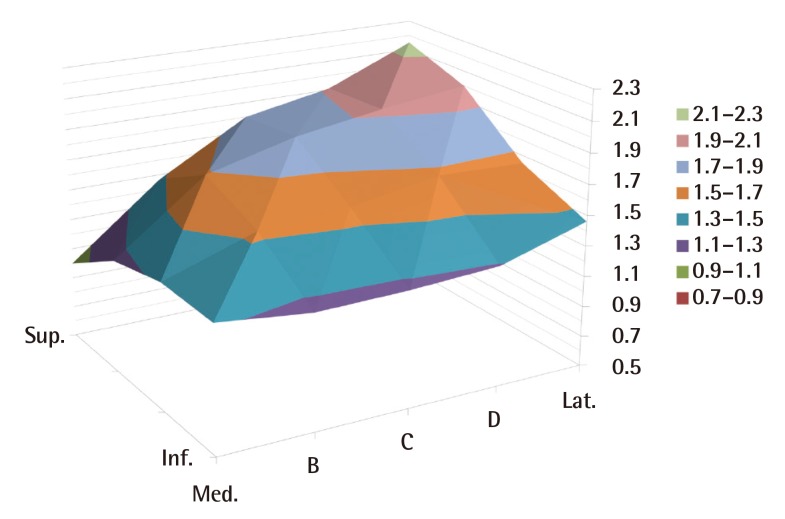
According to the anatomical study on the thickness of the soft tissues, the superomedial flap close to the PSIS was the thinnest among all the specimens. The thickest area was the superolateral area, the mean thickness of which was 3.24 times greater than that of the thinnest area (TI=3.24). The flap tended to be thicker in the direction of the thinnest superomedial area to the inferomedial area than in the superior area. In the inferior area, the difference between the thickness at the medial side and that of the lateral side was comparatively less than in the other areas (Table 1) (Figs. 2, 3).
Three-dimensional graph demonstrating the average TI (cadavar)
TI, measured thickness of each division/thickness of the thinnest division; Sup., superior; Inf., inferior; Med., medial; Lat., lateral.
Biotrepy through CT imaging
Thickness measurements of the gluteal soft tissues
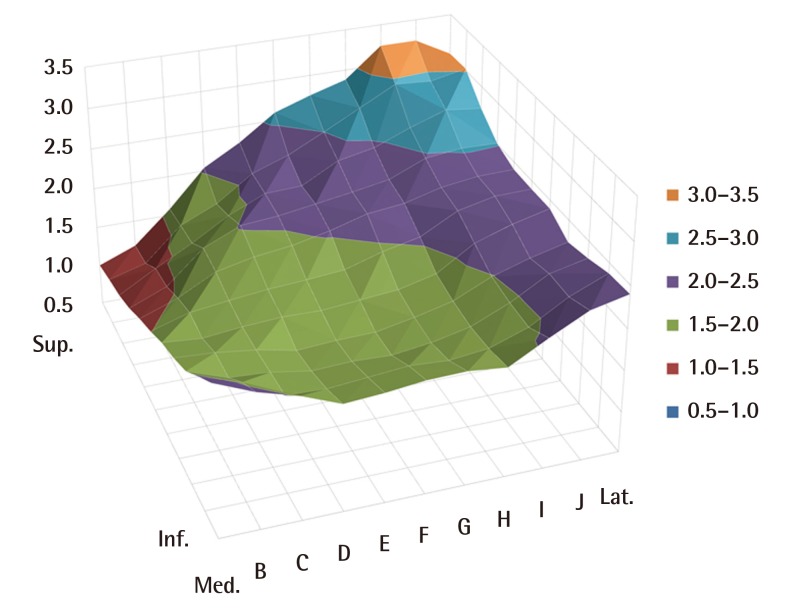
Similar to the aforementioned anatomical study, the CT study showed that the thinnest area of the gluteal soft tissues was around the PSIS (A-1) in the superomedial area of the buttock, and the thickest area was the lateral superior area (E-1), the mean thickness of which was 2.15 times greater than that of the thinnest area (TI=2.15). In the inferior area, similar to the results of the anatomical study, the difference between the thickness at the lateral side and that at the medial side was comparatively less than that in the other areas. The similarity of results between the two studies was confirmed by 3D graphs (Table 2) (Figs. 2, 4).
Statistical analysis
The significant difference in the thicknesses of the soft tissues in the various areas on the CT images was confirmed through a one-way ANOVA test. Other than in the two inferomedial areas (B-4 and C-4), a statistically significant difference in the mean values was observed (Table 3) (P<0.016).
The age, height, weight, and BMI showed no significant correlation with the TI of the thickest area (E-1). The TI of the thickest area and the age showed a low positive correlation, with a correlation coefficient of 0.239 (P=0.009). Weight showed a negative correlation with TI (correlation coefficient, -0.353), as did BMI (correlation coefficient, -0.363) (P<0.001) (Table 4).
DISCUSSION
This is the first study to provide information on the distribution of thicknesses of gluteal soft tissue, which is used for flap surgery. Previous studies on the thickness distribution of the gluteal soft tissues have been conducted to confirm the length of the needle needed for gluteal intramuscular injections [16,17]. Such studies were meant to measure the thickness of a specific area through a CT scan; therefore, the thicknesses of the areas other than that of the intramuscular injection were not measured. There have been several studies on the distribution of the thickness of body areas according to the BMI, including that of the gluteal soft tissues, to confirm the appropriate needle length for subcutaneous self-injection by diabetic patients, but such studies have not provided information for surgery [18].
Studies on the distribution of the thickness of the gluteal soft tissues may help with flap surgery planning. Since relapse is common with sacral pressure sores, sufficient blood flow and padding should be provided to the affected area; therefore, the donor site and the design of the flap can be chosen accordingly. The frequently used perforator flap has the advantages of being able to accomplish full-scale functional reconstruction without sacrificing muscles, being able to provide sufficient blood flow and soft tissues, and having an available alternative for secondary surgery in cases of recurrent pressure sores. In the design of the gluteal perforator flap, the location of the perforator is the major factor that must be considered. To obtain thick and strong soft tissues, a blood supply vessel can be selected from multiple perforators in various locations, after which the locations and shapes of the flaps must be chosen. For example, in sacral pressure sore reconstruction, superior buttock arterial perforators are often found at the medial two-thirds of the line that connects the PSIS to the GT [3]. According to the results of the present study, the sacral area can be used if the thick tissues in the lateral superior area are selected to obtain arterial perforators that can serve as propeller-type flaps for the affected area (Fig. 5). Among the various flap procedures such as rotation, advancement, and displacement, the propeller-type flap procedure uses the thick superolateral tissues for medial side defects. This method not only provides patients with an abundant cushion but also helps with wound healing because the donor site is far from the pressure sore area and has healthy tissues.
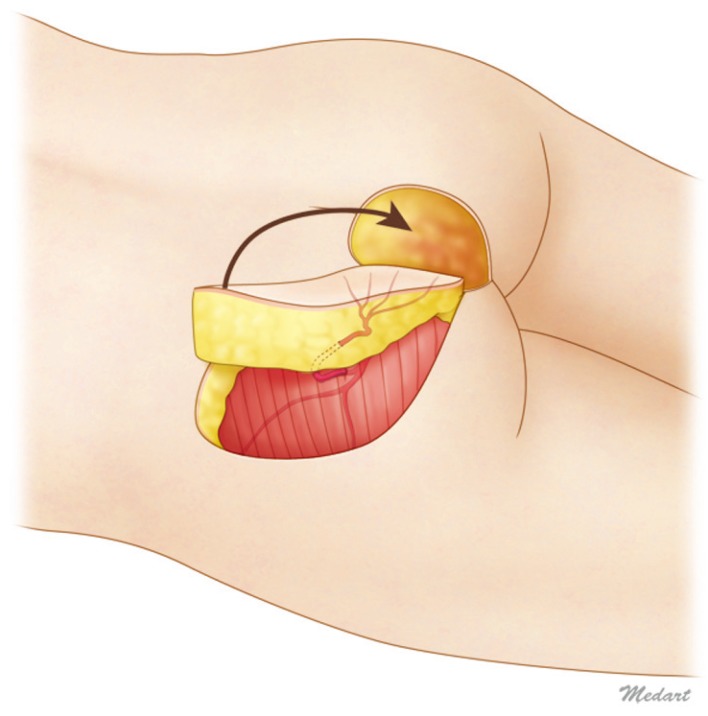
Ideal gluteal perforator flap design
Because the superolateral part of the buttock has the thickest soft tissue volume, the superior gluteal artery perforator propeller flap can provide adequate padding for sacral sore reconstruction.
The most frequently used autologous tissues in breast reconstruction are obtained from the abdomen. However, because of insufficient fat tissues in the abdomen, a history of surgery, or patient preference, free flaps from the gluteal arterial perforators may be used [11]. Since the first free flaps that included muscles were used, the perforator flap was widely used from 1995, when Allen and Tucker [19] used superior buttocks arterial perforator flaps for breast reconstruction [5]. In 2004, a method that involved the use of the inferior buttocks arterial perforator was introduced [10]. Breast reconstruction using gluteal perforator flaps is advantageous in terms of its abundant fat volume, concealable donor site scar, and natural 3D reconstructed breasts. Various designs have been suggested according to the location of the flap donor site and the flap shape. Using the superior or inferior area of the buttocks, superior or inferior buttock arterial perforator flaps, oval flaps, or crescent-shaped flaps are designed. In the design of flaps for breast reconstruction, various factors such as the shape of the healthy breast, symmetry, and patient preference are considered. In addition, abundant volume is required in the inferolateral area of the reconstructed breast, and comparatively less volume is necessary in the superomedial area. However, previous studies have focused on the location of the perforator or the changes in the donor site shape. If the distribution of the thickness of the gluteal soft tissues is considered with the other factors described in this study, better designs may become available. In 2008, Kronowitz [11] suggested a design that was based on the consideration of the thickness of a flap for 3D breast reconstruction. According to Steven's oval design, the superolateral gluteal area that was the thickest buttock area was used for the inferolateral area of the breast, and the thin medial side flap was used for the superior area. Steven obtained cosmetically superior results with reduced donor morbidity using the design. His design was for an ideal flap that was based on the consideration of the thickness distribution in the donor site. The volume and location of the perforator are determined according to the body shape, severity, and location of defects, and the shape of the opposite breast of the patient. If the information on the mapping of the thickness of the gluteal soft tissues reported in this study is used, a customized ideal flap design may be possible.
In this study, correlations between the TI and factors such as age, weight, and BMI were confirmed. Age showed a weak positive correlation with the TI, but the weight and the BMI showed a negative correlation. In pressure sore patients, fat mass often decreases due to causes such as nutritional insufficiency. Thus, for such patients, it is preferable to use the superolateral gluteal area as a thick flap rather than the medial side area.
For the anatomical study, typical fixatives and fixed cadavers were used. During the fixation process, the gluteal medial side area might have been transformed due to weight pressure. To correct for this transformation, the flaps were fixed again in a flat-bottom box for ten days after they were separated. The re-fixed flap surface had an almost even thickness and a negligible pressure transformation. The difference in the fat thickness among the areas analyzed was confirmed visually taking into account the distribution and density of fat (Fig. 3). When a CT scan was conducted, the supine position of the patients might have affected the gluteal shape, but the cushion of the bed that was provided to them was curved and the gluteal pressure was deconcentrated; therefore, the effect was considered negligible. For further studies, fresh cadavers and patients' prone CT images may need to be compared.
The deposition area and the amount of fat tissue may vary according to the patient's ethnicity and cultural background, overall fat mass, and body shape [20,21,22,23]. Asians have less abundant lateral side buttocks and lateral side thighs with smaller buttocks than Hispanics, Caucasians, and Africans. Because different ethnic groups have different skeletomuscular shapes, the fat layers that form the gluteal shape also differ among ethnic groups. Accordingly, the results of this study may not correspond with those of other studies. In this study, comparatively diverse adult age groups and different genders were selected from the same ethnic group with the same cultural background. Additional studies with various ethnic groups may be required in the future.
Through anatomical and CT imaging studies, mapping of the thickness of Korean gluteal soft tissues was attempted in this study. The mapping information may help determine the optimal flap design in pressure sore reconstruction using local flaps and in breast reconstruction using free flaps. For sacral pressure sore reconstruction, superolateral soft tissues are recommended as the donor site for the propeller-type flap. For breast reconstruction, the gluteal superolateral area should be used for the inferolateral area of the breast, and the thin tissues of the gluteal medial side should be used for the superior area of the breast for a favorable 3D breast shape. Appropriate design of the fasciocutaneous flap based on the gluteal perforator may require consideration of not only the location of the perforator but also the thickness of the area of fat tissues.
Notes
This article was presented at the 70th Congress of the Korean Society of Plastic and Reconstructive Surgeons on November 9-11, 2012, in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.