Nanotechnology Biomimetic Cartilage Regenerative Scaffolds
Article information
Abstract
Cartilage has a limited regenerative capacity. Faced with the clinical challenge of reconstruction of cartilage defects, the field of cartilage engineering has evolved. This article reviews current concepts and strategies in cartilage engineering with an emphasis on the application of nanotechnology in the production of biomimetic cartilage regenerative scaffolds. The structural architecture and composition of the cartilage extracellular matrix and the evolution of tissue engineering concepts and scaffold technology over the last two decades are outlined. Current advances in biomimetic techniques to produce nanoscaled fibrous scaffolds, together with innovative methods to improve scaffold biofunctionality with bioactive cues are highlighted. To date, the majority of research into cartilage regeneration has been focused on articular cartilage due to the high prevalence of large joint osteoarthritis in an increasingly aging population. Nevertheless, the principles and advances are applicable to cartilage engineering for plastic and reconstructive surgery.
BACKGROUND
There are three different forms of cartilage in the body: hyaline, elastic and fibrous cartilage. Each can be found in specific sites and with different properties and functions. Hyaline cartilage can be found in the joints, nose, trachea and ribs [1]. In adults, its firm yet pliable character provides a shock-absorbent effect in the mechanical loading of joints, and a stent-like support to the nose and trachea, whilst allowing flexibility and recoil to the respiratory system during breathing [2]. Elastic cartilage, as its name implies, provides elastic properties to the epiglottis, external acoustic meatus, Eustachian tubes and external ears [3]. Fibrous cartilage, characterised by an abundance of matrix collagen type I fibres, is present in intervertebral discs, the menisci of the knees, the symphysis pubis, and the intra-articular discs of the sternoclavicular and temporomandibular joints [3].
Adult cartilage is predominantly an avascular, aneural and alymphatic tissue. It inherently lacks the ability to regenerate following loss from disease or degeneration. Significant cartilage defects are seen clinically in congenital anomalies such as microtia, following facial trauma, excision of tumours of the respiratory tract, and in degenerative osteoarthritis. However, current reconstructions based on autografts, allografts, implants or prostheses can be less than ideal. Autologous donor sites are very limited; allografts are associated with the risks of disease transmission and immunosuppression, implants with the risks of infection and extrusion, and prostheses with limited functionality. The field of cartilage engineering has emerged in the search for a functional cartilage replacement to meet specific clinical requirements.
Tissue engineering is a multidisciplinary field that applies the knowledge of engineering and biological sciences in the development of biological substitutes to restore, maintain or improve tissue functionality [4]. That function is influenced by four fundamental components: 1) cells, 2) scaffolds, 3) bioactive cues, and 4) environmental factors. The production of cartilage extracellular matrix (ECM) requires either chondrocytes or mesenchymal stem cells. A biocompatible three-dimensional (3D) scaffold would provide the foundation for the cells to adhere, proliferate and then be delivered for transplantation. Bioactive cues such as growth factors and arginine-glycine-aspartate (RGD) peptide sequences are essential in signalling and inducing cells to proliferate and produce the appropriate ECM components. Chondrocyte survival and function is largely influenced by environmental conditions such as pH, oxygen tension and dynamic mechanical induction [5,6,7].
To date, detailed cartilage regeneration studies of human hyaline cartilage have been predominantly focused on articular cartilage rather than nasoseptal, auricular or costal cartilage. This has been driven by the volume of demand related to degenerative osteoarthritis [5,8]. Articular cartilage samples have been more widely available to science due to the prevalence of joint replacement surgery. Nonetheless, the fundamental principles and advances of cartilage regeneration derived from articular cartilage studies provide a template for the engineering of head and neck cartilage. This article provides a brief overview of native cartilage composition and structural architecture, as well as highlighting the evolution and recent advancements in scaffold technology. Emphasis will also be given to state of the art strategies in biomimetic nanotechnology scaffold development for cartilage regeneration.
CARTILAGE EXTRACELLULAR MATRIX STRUCTURE
The cartilage ECM consists of an integrated 3D fibrous network of structural macromolecules, which are produced and organised by chondrocytes bathed in interstitial fluid (Fig. 1). Water constitutes up to 80% of the wet weight of cartilage while the remainder consists of macromolecules-predominantly collagen and proteoglycans [9]. Collagens constitute more than 60% of the dry weight of cartilage, while proteoglycans and other non-collagenous proteins contribute 25% to 35% and 15% to 20%, respectively [9]. Collagen types II, VI, IX, X, and XI are all found in native cartilage, but type II forms the majority of the structural framework, accounting for up to 90% to 95% of collagen content [9,10]. Aggrecan is the predominant proteoglycan in cartilage, accounting for 35% of its dry weight and consists of multiple glycosaminoglycan (GAG) chains, which are chondroitin sulphate and keratin sulphate chains linked to a protein backbone [9,11]. Most aggrecans associate non-covalently with hyaluronan to form high molecular weight proteoglycan aggregates [12] and fill most of the interfibrillar spaces of the collagenous network. As these GAG chains are highly hydrophilic and polyanionic, they attract water molecules. This leads to a high osmotic and swelling pressure within the collagenous network. This provides native cartilage the ability to withstand mechanical forces [10,13]. An understanding of the composition and structural organisation of native cartilage is essential to the design of biomaterials and methods of nanofibrous scaffold fabrication for cartilage tissue engineering.
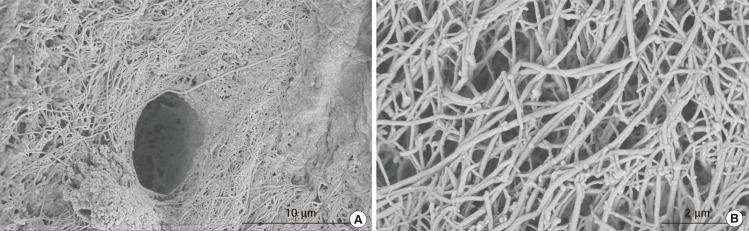
Human hyaline cartilage extracellular matrix structural architecture
These are scanning electron microscopy (SEM) images of a section of human hyaline cartilage. (A) A lacuna surrounded by a dense collagen framework. (B) A high resolution image of cartilage extracellular matrix and collagen fibres. These SEM images of native human hyaline cartilage were fixed in 2.5% glutaraldehyde, followed by step-wise dehydration and sputtered gold coating.
APPLICATIONS FOR BIOMATERIALS AND THE EVOLUTION OF SCAFFOLD TECHNOLOGY
To date, several natural and synthetic biomaterials have been investigated as tissue regenerative scaffolds. They include alginates [14], chitin [15], collagen [16,17,18], elastin [16], hyaluronan [19], and synthetic polymers such as poly(glycolides) (PGA) and poly(lactides) (PLA) [20,21]. Natural polymers have the advantage of being biocompatible and contain more bioactive cues and signalling molecules to facilitate cell attachment and/or maintain differentiation [4]. However, many natural materials suffer batch variability, limited large-scale availability [4], high cost and potential immunogenicity. Synthetic materials can be versatile in their physical and chemical properties, and can be tailored to allow precise control over attributes such as molecular weight, degradation time, and hydrophobicity [22]. The advantages of both natural and synthetic materials can be combined within strategies for scaffolds where critical amino acid sequences or components from natural polymers are incorporated [17,23]. A well-established example of this in plastic and reconstructive surgery is the Integra Dermal Regeneration Template (Plainsboro, NJ, USA), in which bovine type I collagen and chondroitin sulphate has been cross-linked to a poly(dimethylsiloxane) (PDMS) membrane to form a bilaminar silastic construct for the treatment of burns and scars (Fig. 2A, B) [24,25]. The PDMS layer acts as an epidermal barrier analogue to control fluid loss and prevent pathogen invasion, while the co-cell type I collagen-chondroitin sulphate layer serves as an intermediate matrix for soft tissue and vascular integration.
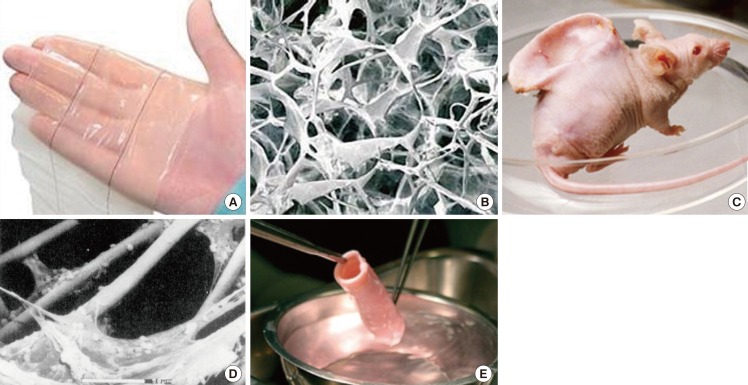
Pioneering tissue engineered skin and cartilage constructs
(A) Gross appearance of Integra, (B) Scanning electron microscopy image of the type I collagen-chondroitin sulphate scaffold component of Integra (Permission granted by Integra LifeSciences Corp., Plainsboro, NJ, USA). (C) Human ear-shaped PLA-coated PGA scaffold seeded with bovine articular chondrocytes and implanted into the dorsum of an athymic nude mouse (BBC Photo Library), (D) Scanning electron microscopy of the cell-scaffold construct prior to implantation. Note the micron-sized PGA fibres. Scale bar=50 µm (Reprinted from Cao et al. Plast Reconstr Surg 1997;100:297-302, with permission from Lippincott Williams and Wilkins [27]). (E) Pre-implanted segment of tissue-engineered allogeneic airway, which was decellularised and reseeded with the recipient's bronchial epithelial cells and mesenchymal stem cell-derived chondrocytes (Reprinted from Macchiarini et al. Lancet 2008:372;2023-30, with permission from Elsevier [29]).
Traditionally, many engineered biomaterial scaffolds were designed and produced to support cell culture and to match the properties of the desired native tissue at a macroscopic level. For example, in the 1980s, the type I collagen-chondroitin sulphate component of Integra was produced by a freeze-drying technique [26] to achieve a highly porous scaffold architecture considered to be microporous (Fig. 2B). In 1997, in pioneering work by Cao et al. [27], bovine articular chondrocytes seeded on a PLA-coated PGA fibrous scaffold moulded into the shape of a human auricle were evaluated using an in vivo mouse model. The fibre diameter of the PGA scaffold was 15 µm, which was almost equivalent to the size of a cell (10-20 µm), and two orders of magnitude larger than a natural ECM fibril (Fig. 2C, D). Because the majority of native ECM (cartilage included) consists of a 3D complex nanoscaled fibrillar network of not only structurally but also functionally integrated nanoscaled structures, the current trend in engineering tissue scaffolds is to mimic the nanofibrous architecture of ECM proteins [5,28]. This is the key difference from preceding strategies that lacked the nanoscaled features of native ECM. The tissue-engineered allogeneic airway transplantation reported by Macchiarini et al. [29] in 2008, further emphasises this argument. The donor airway was decellularized, leaving behind the ECM "skeleton" for subsequent recolonisation with recipient cells (Fig. 2E). This ECM "skeleton" may have provided the recipient cells with a near ready-made environment for cell survival, as indicated by the successful immediate clinical outcome.
THE IMPORTANCE OF NANOSCALE ARCHITECTURE TO SCAFFOLDS
The scaffold architecture governs how cells spread and bind, leading to changes in intracellular signalling pathways, which ultimately result in a modification of gene expression and cell behaviour (Fig. 3) [30]. Cells seeded on microporous or fibrous scaffolds, which are tens of microns in diameter, tend to attach and flatten as though they were cultured on a flat two-dimensional surface. This culture condition leads to imbalanced receptor attachment and unnatural activation of intracellular signalling [31]. It clearly differs from the natural 3D environment of the ECM, which controls cell behaviour and function [30,32]. Nanoscaled fibrous scaffolds, on the other hand, are one to two orders of magnitude smaller than cells, and more closely simulate the natural nanofibrous matrix for cartilage cells, increasing cell binding sites and directing cell behaviour and functionality. Scaffolds with nanoscale architecture also have a higher surface area to volume ratio for the adsorption of proteins and binding of ligands. This means that a higher number of binding sites and guidance cues are presented to cell receptors and has important implications for the biomimicking of the functions of native ECM in scaffold production. Thus, nanoscale fibrous scaffold architecture is crucial in promoting and maintaining chondrogenic differentiation [33,34,35].
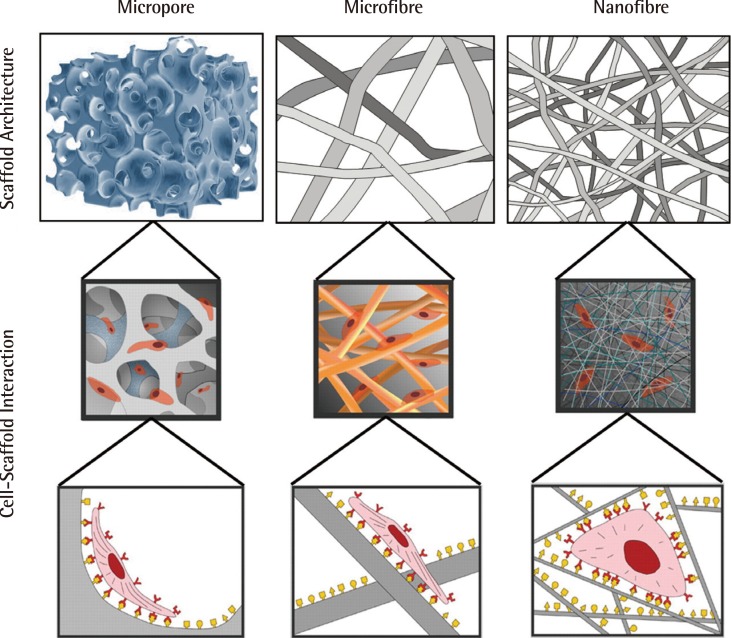
Impact of scaffold architecture on cell behaviour
Scaffold architecture influences cell binding, hence also cell behaviour and function. This figure illustrates the way in which nanoscaled fibrous scaffolds provide an environment for cells which better resemble the fibrous extracellular matrix of cartilage (Reprinted from Stevens and George. Science 2005;310:1135-8, with permission from AAAS [30]).
CURRENT TECHNIQUES FOR THE FABRICATION OF A NANOSCALED FIBROUS SCAFFOLD
Currently, three well-established techniques are used to fabricate nanoscale fibrous scaffolds: self-assembly, phase separation and electrospinning [30,36].
Self-assembly
Self-assembly is a process of autonomous self-organisation of molecules into patterns and structures without human intervention [37]. It occurs commonly throughout nature and technology, mediated by non-covalent interactions such as van der Waals, electrostatic and hydrophobic interactions, as well as hydrogen and coordination bonds [37]. For instance, amphiphilic molecules possessing both hydrophilic and hydrophobic segments, may self-assemble into well-ordered structures when dispersed in aqueous solvents [38]. A naturally occurring example and commonly used biomaterial for tissue culture scaffolds is collagen. Its propeptides self-assemble into procollagen and ultimately nanoscaled fibrils under such intermolecular forces.
Synthetic polypeptide based systems are capable of fabricating nanoscale scaffolds with a fibre diameter typically around 10 nm [39,40], and these have been shown to support the growth of various cell types as well as chondrocytes [41,42,43,44,45]. In cell culture, these scaffolds physically behave like hydrogel systems engulfing the cells in a 3D manner [41,42,43]. This technique produces fibres with diameters typically smaller than those of native ECM (Fig. 4). An advantage of using peptide self-assembly is that the scaffold has the potential to carry many more biologically compatible motifs on its surface compared to other synthetic polymeric biomaterials such as polyesters which lack functional chemical groups. However, the low yield and the complexity of the fabrication procedure make it prohibitive for larger scale tissue engineering [36].
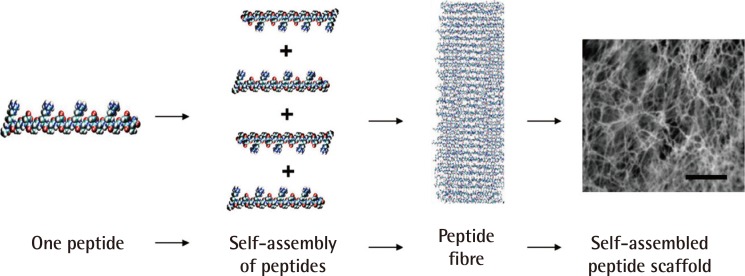
Self-assembly of peptides to form nanofibres
This is an example of a self-assembled peptide used in a study of wound healing. A single peptide, approximately 6 nanometres, is shown. Thousands of peptides self-assemble to form a single nanofibre; trillions of peptides or billions of nanofibres form the scaffold (scale bars=0.5 µm) (Reprinted from Schneider et al. PLoS One 2008;3:e1410 [43]).
Phase separation
Phase separation is a technique for the separation of a polymer solution into polymer-rich and solvent-rich domains, either induced thermally or by the addition of a non-solvent of the polymer to form a gel. This is followed by a cooling process to fix the morphology, and a freeze drying process to remove the solvent. This technique produces a 3D fibrous network with fibre diameters ranging between 50 nm and 500 nm, and with a porosity as high as 98% (Fig. 5) [46]. These scaffolds have been shown to favour cell attachment, exhibit higher adsorption of proteins such as fibronectin and vitronectin, and promote differentiation when compared to solid walled porous scaffolds with equal porosity [47,48]. Scaffolds fabricated from synthetic and natural polymers using phase separation have been shown to support chondrocyte proliferation and enhance cartilage phenotypic expression [49,50,51]. Phase separation offers the ability to control the fibre diameter and the porosity of the scaffold, as well as tailor its mechanical properties by manipulating the processing parameters [36,46]. In addition, since this process of scaffold fabrication takes the shape of its moulds, it enables the scaffolds to be fashioned to any desired anatomical shape [52]. Batch-to-batch consistency can be achieved with this technique, but it requires experience. Although scaffold production with phase separation requires specialised equipment, it lacks the ability to align fibres provided by electrospinning.
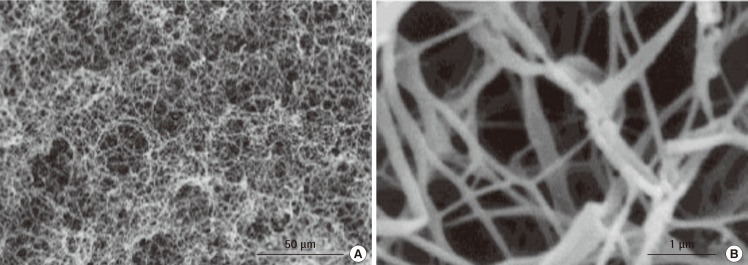
Formation of PLLA scaffolds with phase-separation
Scanning electron microscopy images of poly(L-lactide) (PLLA) scaffolds produced using the phase-separation technique. (A) 500×, (B) 20,000× magnification (scale bars are 50 µm and 1 µm, respectively) (Reprinted from Ma and Zhang. J Biomed Mater Res 1999;46:60-72, with permission from John Wiley & Sons, Inc. [46]).
Electrospinning
Electrospinning is an electrostatic technique which produces non-woven polymer fibres with controlled diameters ranging from a few microns down to a few nanometres. The technique employs an electrical field gradient generated between a spinneret and grounded target collector (Fig. 6). When an electrical field applied across a polymer solution reaches the point where the self-repulsive charges overcome the forces of surface tension of the polymer solution, an accelerating jet of charged polymer solution travels toward the opposing or zero-charged ground target. As the jet travels in mid-air, the solvent evaporates, leaving behind a charged polymer fibre which continues to elongate to the collecting target. Such a technique has numerous and diverse applications because of its ability to produce nanoscaled fibres, technical simplicity and cost effectiveness [36,53]. Since the first patent by Formhals in 1934, electrospinning has found widespread application in the textile industry, air filtration and biomedical applications including wound dressings and drug delivery [53]. It has also become a powerful tool in producing non-woven nanofibrous biomaterial scaffolds in tissue engineering applications [16,20,54].
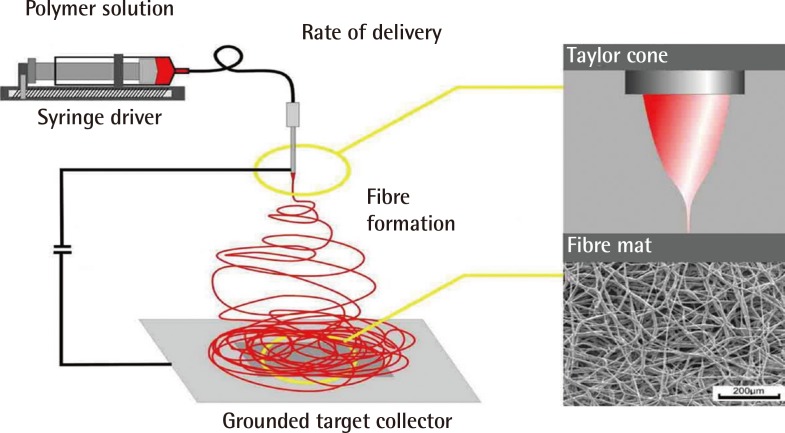
Schematic representation of a typical electrospinning system
The polymer solution is delivered, at a constant rate, to the tip of the charged spinneret. Electrospinning is initiated from the Taylor cone (depicted top right) when the charge overcomes the forces of surface tension. The electrospun fibres are then collected on the grounded targe (Image courtesy of Dr. Julian George, Imperial College London).
This technique has an advantage over self-assembly and phase separation methods because it has the ability to produce fibres which are either randomly orientated or aligned in parallel. This can be achieved by rotating the grounded target collector at different speeds [55]. Aligned natural and synthetic electrospun fibres, such as collagen and polyesters, have been shown to provide topographical guidance to cell attachment, orientating cells along the direction of the fibres [54,56]. One of the great strengths of electrospinning is in mimicking the natural structural architecture of many tissue types such as neural, musculoskeletal, dermal and vascular tissues, where the direction of tissue growth is crucial in determining the appropriate physiological function of the organ. As an example, our group has successfully electrospun a nanoscale fibrous poly(L-lactide) (PLLA) scaffold that appears to resemble the structural architecture seen in the native human hyaline cartilage (Fig. 7).
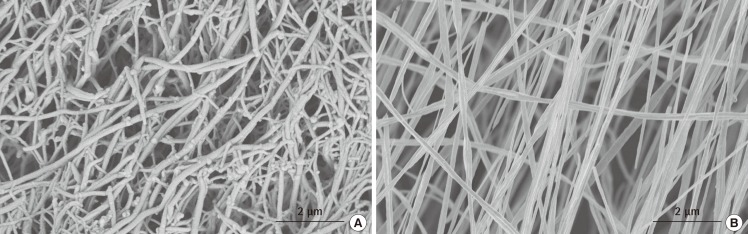
The ability to mimic nature with electrospinning
This is a comparison between the structural architecture of native human hyaline cartilage (A) and electrospun poly(L-lactide) (PLLA) scaffold (B). The fibrous PLLA scaffold was electrospun at a concentration of 3.0% w/w, 10 kV, at a rate of polymer solution delivery of 0.4 mL/hr and target distance of 10 cm.
AN OVERVIEW OF TISSUE SCAFFOLD BIOFUNCTIONALISATION
In engineering biomimetic scaffold design, apart from fabricating the scaffold's physical structure with physical and chemical properties to match the desired native tissue, it is also essential to design for functionality. This process has been referred to as the biofunctionalisation of scaffolds, in particular, those scaffolds that may lack the functional surface chemical properties for cellular interaction. Biofunctionalisation often involves scaffold surface modification followed by physical and/or chemical linkage with biologically active compounds. Scaffold surface modification techniques commonly involve the use of either plasma or chemical treatments [57]. These surface modification techniques introduce reactive chemical groups such as amine, hydroxyl or carboxyl groups, which are recognisable by cells and form a platform for subsequent crosslinking with natural ECM components and bioactive compounds. These bioactive compounds include several native ECM components such as RGD peptide sequences for cell attachment, collagen and chondroitin sulphate, as well as growth factors, e.g., transforming growth factor-β1 (TGF-β1) and basic fibroblast growth factor [23,58,59].
Growth factors play a vital role in the induction, promotion and maintenance of differentiation and the function of cells, particularly chondrocytes, which inherently lack the capacity to regenerate. However, incorporating growth factors in vitro has been challenging. The short half-lives of growth factors such as TGF-β1 (<30 min) and insulin-like growth factors (10-12 min) as well as their dose-related adverse effects have persuaded researchers to incorporate growth factors into scaffold designs [60]. The controlled release of growth factors can also be tailored into biomimetic scaffold design [61]. Several methods have been described [60]: 1) by direct blending or emulsion of the growth factors with the biomaterial during the scaffold synthesis and fabrication stage, 2) by physical adsorption through the immersion of the scaffold in a growth factor solution, and 3) by pre-chemical linkage to or encapsulation by a carrier prior to step 1). For example, poly(ether-ester) copolymer emulsion containing TGF-β1 has been used to coat porous PEG-based scaffolds [62]. This system was seeded with goat bone marrow-derived mesenchymal stem cells and shown to produce glycosaminoglycans, indicating the induction of the stem cells towards a cartilage lineage. The release of the growth factors is typically governed by the degradation rate of the scaffold material. Elisseeff et al. [63], encapsulated Insulin-like growth factor-I and TGF-β1 within PLGA polymer microspheres using a double emulsion technique. Together with bovine chondrocytes, these microspheres were then blended within PEG-based hydrogel scaffolds. The chondrocytes were shown to proliferate and increase matrix glycosaminoglycan production in response to the cues received from the biofunctionalised scaffolds [63]. These examples of biofunctionalised scaffold systems have demonstrated that growth factors were released from the scaffolds and induced changes in cell behaviour.
CONCLUSIONS
Tissue engineering has advanced over the past two decades and continues to evolve in search of optimal tissue replacements alongside nanotechnology. The concept and results of mimicking the structure and function of the natural ECM form the current direction of travel for the fabrication of an optimal tissue regenerative scaffold.
Although the results of current studies have been encouraging, further refinements need to be made. As active growth factors used in current studies are inevitably subjected to contact with organic solvents or time-consuming procedures during processing and scaffold fabrication, it is likely that the majority of the growth factors are denatured. Uncompromised delivery of any growth factor at an optimal concentration with precise release kinetics is ideally required to translate growth factor delivery from an in vitro to in vivo level for tissue regeneration. A system of cell-mediated activation of available bioactive molecules may provide a breakthrough. This might be achieved by incorporating the latent form of the desired protein into the scaffold design. The incorporation of nanotechnology and bioactive cues into tissue scaffold design should prove increasingly promising in cartilage engineering.
Many research studies in cartilage tissue engineering often focus on specific areas of interest with encouraging results, but these studies often lack the holistic requirements to produce a successful tissue replacement. Thus, a multidisciplinary collaborative approach which includes specialised stem cell culture, nanotechnology and bioactive cues, materials science, environmental and mechanical stimulation, and bioreactor culture as well as vascular tissue engineering may offer a breakthrough in functional cartilage regeneration.
Notes
No potential conflict of interest relevant to this article was reported.