Effects of the Diabetic Condition on Grafted Fat Survival: An Experimental Study Using Streptozotocin-Induced Diabetic Rats
Article information
Abstract
Background
Autologous fat grafts have been widely used for cosmetic purposes and for soft tissue contour reconstruction. Because diabetes mellitus is one of the major chronic diseases in nearly every country, the requirement for fat grafts in diabetes patients is expected to increase continuously. However, the circulation complications of diabetes are serious and have been shown to involve microvascular problems, impairing ischemia-driven neovascularization in particular. After injection, revascularization is vital to the survival of the grafted fat. In this study, the authors attempted to determine whether the diabetic condition inhibits the survival of injected fat due to impaired neovascularization.
Methods
The rat scalp was used for testing fat graft survival. Forty-four seven-week-old male Sprague-Dawley rats were allocated to a diabetic group or a control group. 1.0 mL of processed fat was injected subcutaneously into the scalp of each rat. The effect of diabetes was evaluated by calculating the volume and the weight of the grafted fat and by histologically analyzing the fat sections.
Results
The surviving fat graft volume and weight were considerably smaller in the diabetic group than in the control group (P<0.05), and histological evaluations showed less vascularity, and more cysts, vacuoles, and fibrosis in the diabetic group (P<0.05). Cellular integrity and inflammation were not considerably different in the two groups.
Conclusions
As the final outcome, we found that the presence of diabetes might impair the survival and the quality of fat grafts, as evidenced by lower fat graft weights and volumes and poor histologic graft quality.
INTRODUCTION
Autologous fat grafts are widely used for cosmetic purposes, for correcting soft tissue contour defects, and for reconstruction, mainly because of their straightforwardness and relatively few associated problems. The major drawback of this procedure is the high absorption rate of the injected fat, which can be 20% to 70% by volume [1].
On the fourth day after injection, new angiogenesis from the host vascular network causes graft revascularization [2], but this process is rather limited to the fat cell mass periphery and centrally located adipocytes have little contact with the vascular supply. Accordingly, the death and removal of the fat cells continue until the reorganized blood supply matches the graft vascular demands, and thus, early and appropriate revascularization is important for graft survival [3,4].
Diabetes mellitus is one of the major chronic diseases in nearly every country. The universal prevalence of diabetes among adults (aged 20-79 years) was 6.4% in 2010, and this percentage is expected to increase to 7.7% by 2030 [5]. Therefore, the number of diabetic candidates requiring an aesthetic fat graft will also gradually increase. However, the circulation complications of diabetes are serious and have been shown to involve microvascular problems, particularly with respect to the impairment of collateral vessel formation [6]. Furthermore, many experimental studies suggest that diabetes impairs ischemia-driven neovascularization.
The principal assumption of this study was that the diabetic condition inhibits the survival of the grafted fat by impairing neovascularization. To investigate this assumption, a rat scalp fat graft model was designed to evaluate the fat survival. The effect of the presence of diabetes on the grafted fat was estimated by calculating the volume and the weight of the grafted fat and by a performing histological analysis on the grafted fat sections.
METHODS
Fat tissue preparation and processing
The fat used in this study was harvested from a 35-year-old patient with no history of any underlying disease, who underwent reduction mammoplasty under general anesthesia. Written informed consent was procured before the operation. Approximately 40 g of adipose tissue was obtained (Fig. 1) and this was washed extensively with phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO, USA) and cut into pieces of less than 1 mm in all dimensions by using a no. 10 blade. The fat was processed under sterile conditions by placing it on an operating room sterile cotton towel, which allowed the absorption of fluids. During this process, the fat was exposed to air for almost 10 minutes. Plasma and cell debris were removed by centrifuging a fat suspension for 3 minutes at 3,000 rpm at room temperature.
Animals
Forty-four seven-week-old male Sprague-Dawley rats weighing 200 to 220 g (Orient Bio Comp., Seongnam, Korea) were used as a rat model for human fat tissue grafts. During the study, the rats were bred under a constant laminar airflow and given standard laboratory chow and water. The rats were used after a two-day adaptation period. The scalp was used as the recipient site for all fat injections [7]. The Animal Care and Use Committee of our institution consented to the experimental protocol.
Streptozotocin-induced diabetes
The rats were randomly divided into two groups (a diabetic group [n=24] or a control group [n=20]). Diabetes was induced by a streptozotocin (STZ) injection. All of the rats were totally fasted for 24 hours before injection. The animals in the diabetic group were injected once with STZ (60 mg/kg, intraperitoneally, Sigma-Aldrich) dissolved in a citrate buffer (0.1 mol/L, pH 4.5, Sigma-Aldrich) [8]. The rats in the control group were injected with an equal volume of the citrate buffer. Diabetes was confirmed as a blood glucose level >300 mg/dL at 3 days after the STZ injection [8]. Blood glucose levels were checked using the One Touch Ultra Glucometer (Accu-chek, Roche Bioproducts, Basel, Switzerland) twice weekly.
Fat injection
Processed fats were transferred into 1.0 mL syringes. Before fat injection, Zoletil (Tiletamine+Zolazepam [1:1], 30 mg/kg; Virbac Korea Inc., Seoul, Korea) and Rompun (xylazine, 10 mg/kg; Bayer Korea Inc., Seoul, Korea) were injected intraperitoneally. After achieving anesthesia, 1.0 mL of fat was injected subcutaneously into the scalp of each rat with a 16-gauge needle (Fig. 2). No post-procedural analgesics or antibiotics were administered to any animal over the 10-week study period, during which animals were checked daily for clinical signs of immune rejection and infection and for behavioral problems.
Fat graft dissection and analysis
At the end of the 10-week study period, the rats were euthanized with CO2 gas, the fat pads were excised and their weights and volumes determined; volumes were obtained using the liquid displacement method. In addition, fat grafts were sent for histological examination. In brief, sections of fat pads were stained with hematoxylin and eosin first and inspected under a light microscope. The histology slides were examined for the following parameters: 1) the presence of intact and nucleated lipocytes; 2) the presence of cysts and vacuoles; 3) degree of inflammation, as evidenced by lymphocyte and macrophage infiltration; 4) vascularity; and 5) collagen and elastic fibrils, which indicated the presence of fibrosis and connective tissue components [1,9]. Each parameter was scored with a semi quantitative scale ranging from 0 to 5. Scores were allocated on the basis of the relative presence of the histological parameters (0, absent; 1, minimally; 2, minimally to moderately; 3, moderately; 4, moderately to extensively; and 5, extensively) [1]. All sections were scored independently by three blinded investigators. Further, CD31 (Abcam, ab24590; Cambridge, England) immunostaining was performed to confirm vascularity.
Statistics
Fat graft weights and volumes were analyzed using the independent samples t-test, and the Mann-Whitney nonparametric test was used for analyzing the ranked histological parameters. The analysis was completed with PASW ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA), and statistical significance was accepted for P-values <0.05.
RESULTS
Body weights and blood glucose levels
During the 10-week study period, each rat was weighed weekly and blood glucose levels were checked twice weekly using One Touch Ultra Glucometer by tail punching. At the end of the 10-week study period, the mean body weights in the diabetic and control groups were 298.5 g and 567.4 g, respectively. Blood glucose levels in the diabetic group were maintained between 300 mg/dL and 500 mg/dL in 21 rats. The other three rats exhibited excessive hyperglycemia (blood glucose >500 mg/dL) and their body weights continuously decreased. One of the three died 1 week post-injection. The other 2 rats died of hyperglycemia 1 month post-injection. We dissected the scalp area to search for immune rejection, but there was no evidence of infection or any immune rejection symptom such as redness, discharge, hemorrhage or necrosis. The rest of the rats were well and survived until the date of euthanasia at 10 weeks after the fat injection.
Gross examination
1.0 mL of processed fat was injected into each rat. The fat graft in the 41 surviving rats remained as a well-defined subcutaneous lump (Fig. 2). No gross evidence of acute inflammation, seroma collection, abscess formation, or tissue necrosis in the grafts or adjacent tissues was observed.
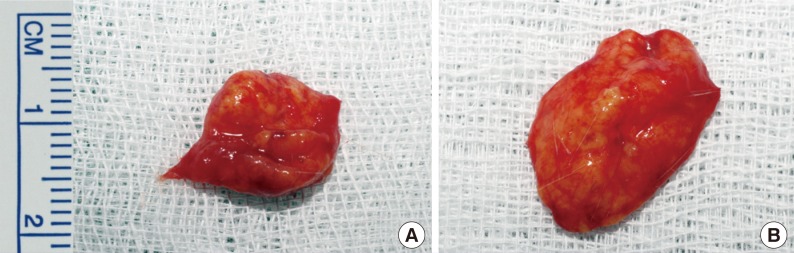
After dissection, grossly all of the fat grafts in the diabetic group rats were half resorbed at 10 weeks post-injection. In contrast, fat grafts in the control group remained intact and appeared as well-defined subcutaneous lumps (Fig. 3).
Intra-dissection photographs of the rat scalp
Intra-dissection photographs of (A) the diabetic group and (B) the control group. Fat grafts in the diabetic group rats showed a half-resorbed state 10 weeks after transplantation. In contrast, the fat grafts from the control group rats were still intact; these grafts existed as well-defined subcutaneous humps.
The average fat graft volumes were 0.413 mL and 0.745 mL in the diabetic and control groups, respectively, which was significant (P<0.05) (Fig. 4), and the average fat graft weights were 0.329 g and 0.651 g, respectively, which was also significant (P<0.05).
Histological evaluations
Analysis of the histological findings of hematoxylin and eosin-stained specimens revealed significantly more cysts and vacuoles in the diabetic group (P<0.05). Further, fat grafts were significantly less vascularized and showed significantly more fibrosis in the diabetic group (P<0.05) (Fig. 5). Although the two groups were not significantly different in terms of the cellular integrity of fat cells and inflammation, diabetic group sections exhibited more inflammation and less fat cell integrity.
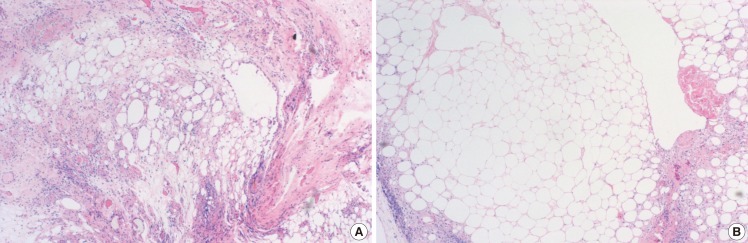
Histological findings of the diabetic and control groups
Fat specimens from (A) the diabetic group and (B) the control group. Fat grafts were sectioned, stained with H&E, and imaged with a light microscope (×100). Irregularly sized fat cells can be seen in the diabetic group, with multiple cysts, vacuoles, fibrous tissues and inflammatory cells, while evenly sized fat cells are visible in the control groups, with few cysts, vacuoles, and inflammatory cells.
The sections of graft taken at 10 weeks were also stained with CD31 antibodies (Fig. 6), and the results obtained showed that fat grafts in the diabetic group were obviously less vascularized.
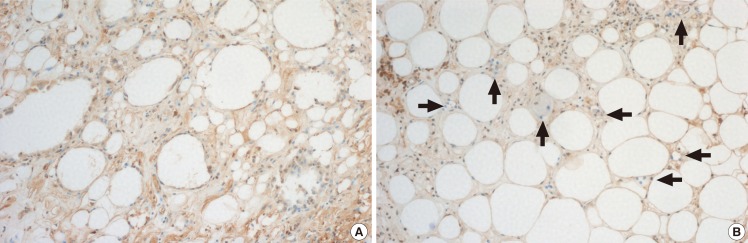
Histological findings of the CD31 antibody stain
The fat grafts were sectioned, stained with CD31 antibodies, and imaged with a light microscope (×100). (A) Histological findings of the diabetic group. (B) Histological findings of the control group. The diabetic group that irregularly sized fat cells exist with vacuoles, fibrous tissues and few vessels surrounded by the intact fat cells. The control group shows evenly sized fat cells with numerous vessels surrounded by intact fat cells. The black arrows indicate vessels. This stain prominently showed that the diabetic group fat graft was less vascularized than the graft in the control group.
The final fat volume, weight, and histologic results are compared in Tables 1, 2.
DISCUSSION
Fat injection for reconstruction and cosmetic purposes was first described in the late 1980s [10], but it was not until the 1990s, when Coleman [11] outlined a standardized reproducible "process" for fat harvesting, centrifugation, and injection, that the reliability and reproducibility of facial "structural fat grafting" became evident. Subsequently, the use of free autologous fat grafts became immensely popular within the medical field, principally because fat tissue is plentiful, easily harvested, and hypo-immunogenic, and also has favorable physical features [12], which include biocompatibility, versatility, a natural appearance, and low donor site morbidity. The major drawback of this procedure is the high absorption rate of the injected fat, which is probably the result of ischemia and a lack of neoangiogenesis [13].
Contradictory theories have been proposed regarding volume maintenance after fat grafting [14]. The most commonly established of these theories is Peer's cell survival theory [15]. Damage of the grafted fat is processed immediately during and after injection because of the mechanical injures due to the injection force and because devascularization causes ischemic injury. After the fat injection, on the first day, neutrophils infiltrate the graft, followed by macrophages, histiocytes, and multinucleated giant cells. Histologic examinations of free fat autologous grafts indicate minimal fat cell necrosis at 48 hours post-injection, with only partial graft vascularization. On the fourth day post-injection, from the host bed vascular system, neoangiogenesis leads to graft vascularization. However, the process is slightly limited to the peripheral area of the graft, and the central cells can only obtain marginal access. This process of adipocyte death and removal continues until the reorganized blood supply meets the graft vascular demand, and thus, early and appropriate revascularization is crucial for fat survival. Delays in vascular supply to tissues cause adipocyte necrosis, which include cell membrane detachment and the development of fatty cysts and vacuoles in non-vascularized regions [16]. Thus, vascularization is the most essential factor of graft survival.
Diabetes mellitus is one of the major chronic diseases, and patient numbers continue to increase, as changing lifestyles reduce physical activity and increase obesity. The world prevalence of diabetes among adults between 2010 and 2030 is expected to change as follows: a 20% increase in the prevalence is expected in developed countries and a 69% increase in developing countries. Therefore, the number of diabetic aesthetic fat graft candidates is sure to increase.
However, significant proportions of morbidities and mortalities among the diabetic population are due to a slow and poor response to tissue ischemia [17]. When chronic tissue ischemia occurs, poor wound healing results in diabetic ulcers and an increase in the lower extremity amputation rate [18]. On the basis of in vitro evidence of increased levels of apoptosis in high-glucose cultures [19] and in cells exposed to hypoxia, it has been assumed that cells exposed to both hyperglycemia and hypoxia show synergistic increases in apoptosis that are expected to increase tissue damage [20]. In vitro, one study demonstrated that prolonged incubation of diabetic cells in normal glucose cultures did not reverse this phenotype [17]. In one recent study, diabetic cells were isolated from mice in which diabetes had been induced by streptozocin of 4 weeks duration. This implies that 1 month of diabetes and hyperglycemia can cause cellular changes that cannot be reversed by glucose-level normalization. This also suggests that impaired neovascularization and a broken normal wound-healing mechanism will persist even after diabetes is cured. This glycemic memory has been observed clinically in the context of neovascular damage despite strict prolonged glycemic control [21]. Thus, we suggest that further studies be undertaken to improve neovascularization and reduce resorption rates to broaden the use of fat grafts in diabetic patients for aesthetic purposes or chronic wound reconstruction.
In terms of experimental human fat grafting, almost every previous study has used the athymic nude mouse model because of its resistance to xenograft rejection [1,2,8,10,22,23]. However, in the present study, we have focused on the hypo-immunogenic characteristics of fat tissue, and processed fat from a human donor in a traditional maneuver and grafted this into Sprague-Dawley rats. Before performing the main experiment, we performed a pilot study on 5 Sprague-Dawley rats per group to observe an immune rejection response. During the 10-week pilot experimental period, no symptoms of hyper-acute or acute immune rejection, such as, infection, redness, edema, or necrosis, was detected. The results of the main experiment concurred with the findings of the pilot study. Nevertheless, the authors are planning further studies to determine the reason for the immune tolerance of the grafted human fat.
To summarize, the present study shows that the presence of diabetes impairs the survival and quality of fat grafts as evidenced by the lower fat graft weights and volumes and poorer histologic graft qualities in the diabetic group. These findings caution that if a fat graft is unavoidable in a diabetes patient, the possibility of an unfavorable result should be considered. Finally, we recommend that further studies be undertaken to improve neovascularization and reduce the resorption rate to allow the indications of fat grafting for aesthetic purposes or reconstruction to be widened to include diabetic patients.
Notes
No potential conflict of interest relevant to this article was reported.