Functional Reconstruction of a Combined Tendocutaneous Defect of the Achilles Using a Segmental Rectus Femoris Myofascial Construct: A Viable Alternative
Article information
Abstract
The composite anterolateral thigh flap with vascularized fascia lata has emerged as a workhorse at our institution for complex Achilles defects requiring both tendon and soft tissue reconstruction. Safe elevation of this flap, however, is occasionally challenged by absent or inadequate perforators supplying the anterolateral thigh. When discovered intraoperatively, alternative options derived from the same vascular network can be pursued. We present the case of a 74-year-old male who underwent composite Achilles defect reconstruction using a segmental rectus femoris myofascial free flap. Following graduated rehabilitation, postoperatively, the patient resumed full activity and was able to ambulate on his tip-toes. At 1-year follow-up, active total range of motion of the reconstructed ankle exceeded 85% of the unaffected side, and donor site morbidity was negligible. American Orthopaedic Foot and Ankle Society and Short Form-36 scores improved by 78.8% and 28.8%, respectively, compared to preoperative baseline assessments. Based on our findings, we advocate for use of the combined rectus femoris myofascial free flap as a rescue option for reconstructing composite Achilles tendon/posterior leg defects in the setting of inadequate anterolateral thigh perforators. To our knowledge, this is the first report to describe use of this flap for such an indication.
INTRODUCTION
Composite defects involving the Achilles tendon and overlying soft tissue pose a significant challenge for the reconstructive surgeon. At our institution, the composite anterolateral thigh (ALT) flap with vascularized fascia lata has become the workhorse for reconstructing these defects as its versatility, flexibility in skin paddle design, potential for simultaneous tendon reconstruction, and minimal donor site morbidity, make it ideally suited for restoring structural integrity to the Achilles region [1,2]. Despite these advantages, however, elevation of this flap is occasionally challenged by the absence or lack of suitable perforators capable of sustaining cutaneous viability (0.89% to 5.4%) [3,4,5,6]. When discovered intraoperatively, a strategy that incorporates nearby tissues derived from the same source vessel provides a logical alternative for successful reconstruction.
The rectus femoris free flap, in particular, has enjoyed widespread clinical application including functional restoration of upper and lower extremity defects, facial re-animation, and dynamic abdominal wall reconstruction [7,8,9,10]. However, its role in the reconstruction of combined Achilles tendon/posterior leg defects has only seldom been reported, and data regarding functional outcomes with this approach are essentially non-existent. In this report, we describe the composite rectus femoris/posterior rectus fascial free flap as a rescue alternative for reconstructing a complex Achilles defect in the setting of insufficient ALT perforators. To our knowledge, this report represents the first to document the use of a rectus femoris free flap for this indication.
CASE REPORT
A 74-year-old male sustained complete subcutaneous rupture of the right Achilles tendon while ballroom dancing. Initial repair via flexor hallucis longus tendon transfer was performed at an outside institution. The postoperative course was complicated by progressive, recurrent infection, and the patient subsequently presented to our clinic after 2 months with a necrotic, infected tendocutaneous defect of the Achilles. Extensive debridement was performed, on 3 separate occasions, resulting in a 9-cm tendon gap with an overlying soft tissue defect measuring 90-cm2 (Fig. 1). Given the extent of the defect, composite tissue transfer utilizing a combined ALT flap with vascularized fascia lata was proposed.
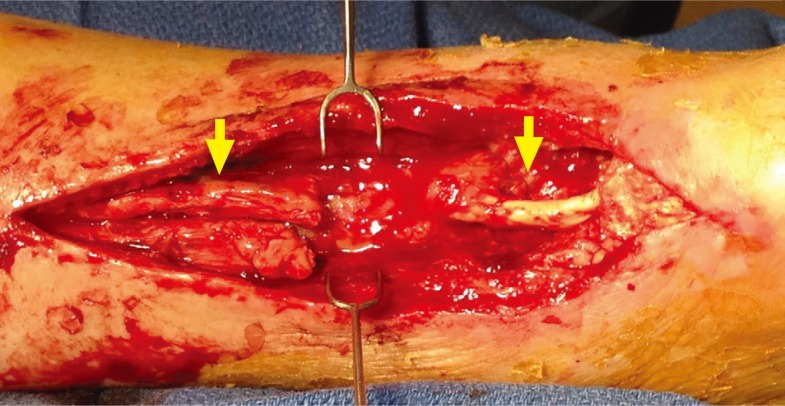
Composite tendocutaneous defect of the Achilles
Segmental defect of the Achilles tendon and associated soft tissue deficit following multiple operative debridements for a failed flexor hallucis longus tendon repair. The tendon gap at the time of definitive reconstruction measured 9-cm with the overlying soft tissue defect measuring 90-cm2. Proximal and distal remnants of the native Achilles tendon can be visualized (yellow arrows).
Doppler examination in the operating theater revealed a weak, yet audible signal along the surface of the proposed ALT donor site. Intraoperatively, however, only a single, small-caliber perforator supplying this region from the lateral circumflex femoral system could be identified. To avoid the additional morbidity of selecting a surrogate donor site, a portion of the thinner, more attenuated rectus femoris muscle was harvested in conjunction with the posterior rectus fascia to reconstruct the soft tissue and tendoachilles defects, respectively (Fig. 2A). End-to-side microvascular anastomosis was performed between the posterior tibial artery and the descending branch of the lateral circumflex femoral artery. The fascial sheet of the composite flap was then rolled into a tendon-like structure and fixed to the proximal and distal remnants of the Achilles, utilizing multiple permanent, non-braided sutures (Fig. 2B, C). The donor site was closed primarily over a suction catheter after plication of the distal extensor mechanism proximal to the patella. A split-thickness skin graft (0.014 inch) was harvested from the ipsilateral thigh for protective coverage of the exposed muscle, and the ankle was immobilized in the neutral position with an external fixation device for 4 weeks (Fig. 2D).
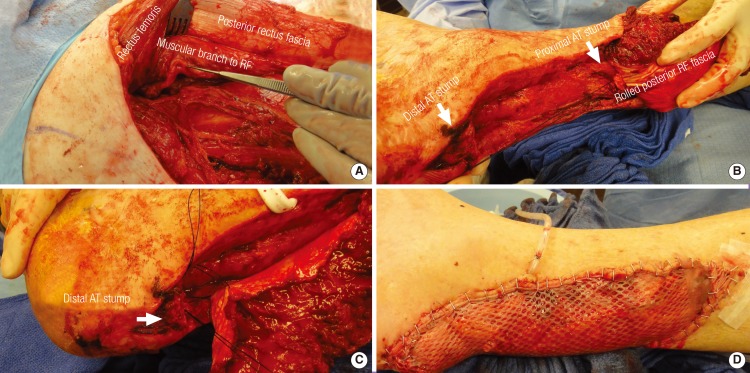
The rectus femoris myofascial free flap
(A) In situ view of the rectus femoris muscle, prior to flap harvest. Partial retraction of the muscle allows for visualization of the posterior rectus fascia as well as the muscular branch to the RF, which is derived from the descending branch of the lateral circumflex femoral artery. (B, C) Transfer and inset of the rectus femoris myofascial free flap into the recipient site. The posterior RF fascia is rolled into a neo-tendon construct and secured to the proximal and distal remnants of the native Achilles tendon (white arrows) using multiple non-absorbable, non-braided sutures. (D) A split-thickness skin graft is harvested from the ipsilateral thigh for protective coverage of the exposed muscle. RF, rectus femoris; AT, Achilles tendon.
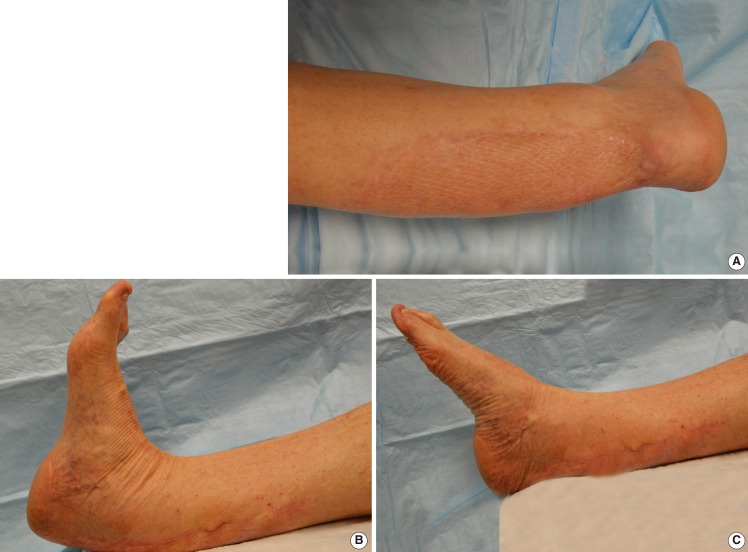
Postoperatively, the patient participated in a graduated rehabilitation program characterized by progression from non-weight bearing range of motion training to pre-gait strengthening and functional re-education with ultimate return to full activity after six months. At the final 1-year follow-up, the patient was ambulating well without support and was able to stand and walk on his tiptoes. He was satisfied with regard to pain, functional recovery, and cosmetic appearance with excellent healing and an absence of scar adhesion on clinical exam. Active range of motion of the affected ankle was 53° (17° dorsiflexion, 36° plantar flexion) compared to 62° (20° dorsiflexion, 42° plantar flexion) for the unaffected side (Fig. 3). Right hip flexion range and knee extension deficit were 0° to 120° and 0°, respectively, comparable to the nonoperative side. There was no clinically noticeable deficiency in quadriceps femoris contraction strength when compared with the contralateral thigh. American Orthopaedic Foot and Ankle Society (AOFAS) and Short Form-36 (SF-36) scores at 1 year improved by 78.8% (52 vs. 93) and 28.8% (80 vs. 103), respectively, compared to preoperative baseline assessments.
DISCUSSION
With advances in microsurgical technique, composite free tissue transfer has emerged as the standard for single-stage, functional reconstruction of complex three-dimensional defects of the distal lower extremity [7]. In our experience with combined Achilles defects, we have found the composite ALT flap with vascularized fascia lata to be a viable alternative for younger, more active patients whose functional demands often necessitate a more normalized range of motion with restoration of power and plantar flexion. However, safe elevation of this flap is occasionally impaired by absent or inadequate perforators supplying the ALT [3,4,5,6]. Use of a hand-held Doppler to map out the perforating vessels may help to identify this situation preoperatively; however, when discovered intraoperatively, alternative options derived from the same vascular pedicle should be pursued. In this report, we describe the use of a composite rectus femoris myofascial free flap to reconstruct a tendocutaneous defect of the Achilles in the setting poorly identifiable ALT perforators.
The rectus femoris, in particular, possesses unique characteristics that make it a versatile option for composite Achilles defect reconstruction. The muscle itself is supplied by 1 or more dominant pedicles based on the lateral circumflex femoral artery and can be elevated as a pedicled or free functional muscle flap, myofascial unit, or myocutaneous flap depending on the unique reconstructive demand [11,12]. When harvested as a free flap in conjunction with the posterior rectus fascia, segmental defects of the Achilles tendon can be reconstructed, simultaneously, using a vascularized fascial construct. This obviates the need to recruit additional surrounding fascia, thereby reducing the complexity of flap dissection as well as the risk of donor site complications.
From an aesthetic standpoint, atrophy and contraction of the denervated muscle flap, over time, provided for an exceptional cosmetic result in our patient. Concern over graft contraction and additional donor site scarring was overcome by harvesting a slightly thicker skin graft followed by excision and primary closure of the donor site, respectively. Overall improvement in preoperative symptomatology and level of function, as well as satisfaction in the aesthetic outcome, appeared to contribute to the high AOFAS and SF-36 scores observed postoperatively.
Despite its unique advantages and broad applicability, however, controversy regarding presumed donor site morbidity has precluded widespread use of the rectus femoris flap. While quantitative studies have shown varying degrees of impairment with respect to voluntary muscle contraction, true muscular capacity, and range of motion, such as knee extension and/or hip flexion, the majority of evidence suggests that these limitations are not clinically significant and are well tolerated by most patients [13,14,15]. Daigeler et al. [14] analyzed the donor site morbidity of 14 patients who underwent pedicled rectus femoris muscle flap coverage for a variety of abdominal wall, groin, and proximal thigh defects. They noted a 21.8% and 18% decrease in maximal voluntary contraction force and true muscular capacity of the remaining quadriceps femoris, respectively. Despite the significant loss of force, however, baseline level of function and active range of motion of the knee and hip were reportedly unaffected. These findings are consistent with those of Gardetto et al. [15] who suggested that harvest of the rectus femoris flap had no clinically significant donor site morbidity and did not adversely affect the patient's capacity for everyday activity.
While we did not quantitatively analyze the contractile force or true muscular capacity of the donor site in this case, full activity was resumed after 6 months without any noticeable functional deficits, thereby confirming the results of previous studies. We attribute this in part to our efforts to centralize the remaining quadriceps femoris by approximating the distal third of the medial and lateral vastus muscles during closure, which has been suggested to assist in the maintenance of donor site biomechanics [13,15]. Furthermore, we advocate for the incorporation of a structured, graduated rehabilitation program, postoperatively, which has been shown to improve donor site strength and stability following harvest of the rectus femoris and is likely a significant contributor to the excellent functional outcomes and lack of appreciable donor site deficits observed in this case [14,15].
In summary, this report represents the first to describe the composite rectus femoris/posterior rectus fascial free flap as a rescue alternative for composite Achilles defect reconstruction. Excellent functional and aesthetic results, high patient satisfaction, and acceptable donor site morbidity make the rectus femoris myofascial free flap a viable alternative for functional reconstruction of the Achilles in the setting of inadequate ALT perforators. As a complement to our reconstructive armamentarium, we now routinely include this option in our preoperative planning and discussion with our patients.
Notes
No potential conflict of interest relevant to this article was reported.