Transposition of Intravascular Lipid in Experimentally Induced Fat Embolism: A Preliminary Study
Article information
Abstract
Background
Liposuction is a procedure to reduce the volume of subcutaneous fat by physical force. Intracellular storage fat is composed of triglyceride, whereas circulating fat particles exist as cholesterol or triglycerol bound to carrier proteins. It is unavoidable that the storage form of fat particles enters the circulation system after these particles are physiologically destroyed. To date, however, no studies have clarified the fatal characteristics of fat embolism that occurs after the subclinical phase of free fat particles.
Methods
A mixture of human lipoaspirate and normal saline (1:100, 0.2 mL) was injected into the external jugular vein of rats, weighing 200 g on average. Biopsy specimens of the lung and kidney were examined at 12-hour intervals until postoperative 72 hours. The deposit location and transport of the injected free fat particles were confirmed histologically by an Oil Red O stain.
Results
Inconsistent with previous reports, free fat particles were transported from the intravascular space to the parenchyma. At 24 hours after infusion, free fat particles deposited in the vascular lumen were confirmed on the Oil Red O stain. At 72 hours after infusion, free fat particles were accumulated compactly within the parenchymal space near the perivascular area.
Conclusions
Many surgeons are aware of the fatal results and undiscovered pathophysiologic mechanisms of free fat particles. Our results indicate that free fat particles, the storage form of fat that has been degraded through a physiological process, might be removed through a direct transport mechanism and phagocytotic uptake.
INTRODUCTION
Liposuction was first introduced in the mid-1970s [1]. Since then, it has become popular and has been widely performed. It is characterized as the fragmentation of the storage form of fat in the body by physical force. During liposuction, the mechanical damage of not only the fat tissue but also the adjacent soft tissue and blood vessels is unavoidable. This causes the fat to enter the damaged blood vessels [2]. The storage form of fat, which is not normally present in the blood vessels, enters the vessels during liposuction. This causes certain pathologic conditions in the human body.
The conditions in which the storage form of fat enters the blood vessels and thereby causes embolism are referred to as fat embolism. If there are clinical signs and symptoms that are associated with fat embolism, the corresponding cases are referred to as the fat embolism syndrome [3]. Housman et al. [4] reported that the mortality due to fat embolism following liposuction is 0.000191% (95 deaths in 496,245 procedures). In addition, there is a remote possibility that the fat embolism syndrome might lead to clinical presentations such as acute respiratory distress, chest pain, nausea, vomiting, and seizure. It is presumed that most patients undergoing liposuction experience subclinical fat embolism, in which there are no clinical symptoms [5].
Even in the body of normal individuals, in cases of the transient rupture of the blood vessels, the storage form of fat may also enter the blood vessels. In these cases, the fat binds to the carrier protein or is removed by phagocytes such as macrophages in the blood vessels. In patients undergoing liposuction, however, a massive amount of abnormal fat abruptly enters the blood vessels. This causes the pathologic conditions of subclinical fat embolism. It is presumed, however, that the sudden intravascular entry of a massive amount of stored fat cannot be managed by carrier proteins or phagocytotic activity.
Given the above background, we conducted this study to examine whether there are any transportation pathways other than the known ones by which the abnormal intravascular entry of the storage form of fat is removed. Prior to conducting in vivo studies, we performed a preliminary animal experiment using rats in a simulation setting for the human body immediately after the liposuction.
METHODS
We performed the current experiment using 24 male Sprague-Dawley rats aged 10 weeks postnatally and weighing 200 g on average (192-211 g). To induce fat embolism in these rats, we infused the human fat aspirates obtained during liposuction. The fat aspirates were centrifuged at 3,000 rpm for 3 minutes. 0.1 mL of supernatant-free oil was mixed with 10 mL of saline. Thus, a 1:100 mixture solution was prepared (Fig. 1). The experimental rats were anesthetized using an intraperitoneal injection of pentothal (20 mg/kg). This was followed by the exposure of the external jugular vein. In the experimental group, we induced fat embolism by using an intravenous injection of a 1:100 mixture solution at a volume of 0.2 mL. In the control group, we infused 0.2 mL of saline (Fig. 2). Some experimental rats (two rats from each group) were sacrificed at 12-hour intervals until 72 hours after infusion. This was followed by a biopsy of the lung and kidney of each experimental rat. At each point in time, the tissue samples of the lung and kidney were stained with Oil Red O and H&E dyes for histopathologic examinations. The samples were then evaluated blindly and independently by one histopathologist.
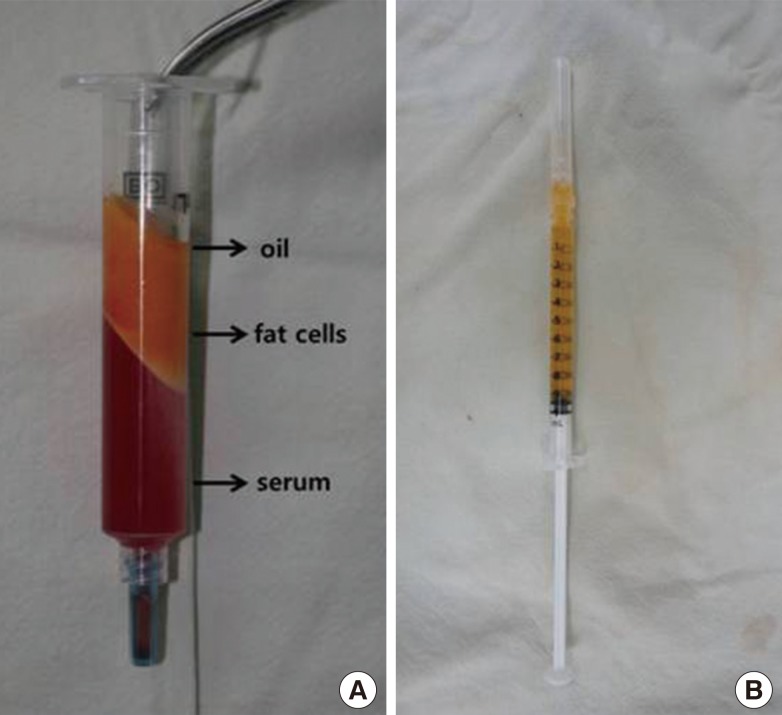
Preparation of the oil solution
(A) The lipoaspirate of human beings after centrifugation. The oil is located in the uppermost layer. (B) The oil is mixed with saline at a ratio of 1:100.
RESULTS
In the control group where only saline was infused, there were no notable findings in the lung and kidney as compared with normal rats.
At 12 hours after infusion, in the lung and kidney specimen, there were red-colored free fat substances on the Oil Red O stain in the blood vessels.
At 24 hours after infusion, free fat substances in the blood vessels were increased more than at 12 hours after infusion. In the lung specimen, there were red-colored free fat substances on the Oil Red O stain in the blood vessels of the lung. In addition, the free fat substances were infiltrated into the vascular wall. On the H&E stain, there were no notable findings other than the normal lung tissue (Fig. 3).
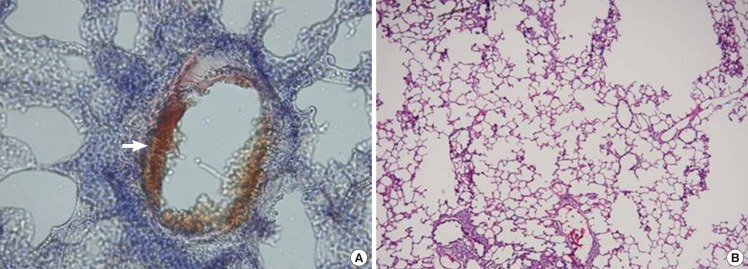
Lung specimen after induced fat embolism
Lung biopsy at 24 hours after injection. (A) Red-colored fat (white arrow) is attached to the vascular wall (Oil Red O stain, ×400). (B) There are no notable findings (H&E, ×200)
At 24 hours after infusion in the kidney specimen, there were also red-colored free fat substances on the Oil Red O stain in the blood vessels of the kidney, as seen in the lung. In addition, there were foam cells around the epithelial cells lining the convoluted tubules on the H&E stain (Fig. 4).
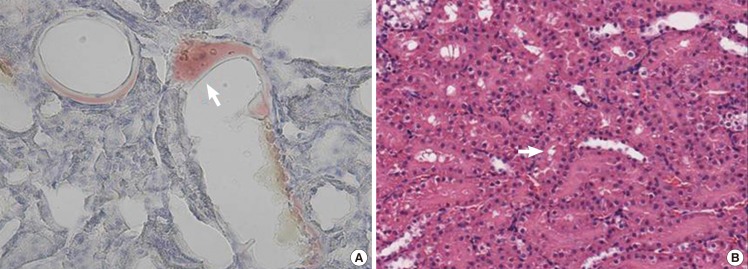
Kidney specimen after induced fat embolism
Kidney biopsy at 24 hours after injection. (A) Red-colored fat (white arrow) is attached to the vascular wall (Oil Red O stain, ×400). (B) Foam cells (white arrow) are observed in the tubular epithelial cells (H&E, ×20).
Free fat substances in the blood vessels decreased gradually after 24 hours and could be observed in the extravascular parenchymal tissue.
At 72 hours after infusion in the lung specimen, unlike at 24 hours, there were red-colored fat droplets on the Oil Red O stain on the biopsy of the rat lung in the extravascular alveolar parenchyma. This confirmed that the intravascular fat droplets permeated the vascular wall and then migrated to the tissue. On the H&E stain, however, there were no structural changes or acute inflammatory reactions suggestive of vascular destruction (Fig. 5).
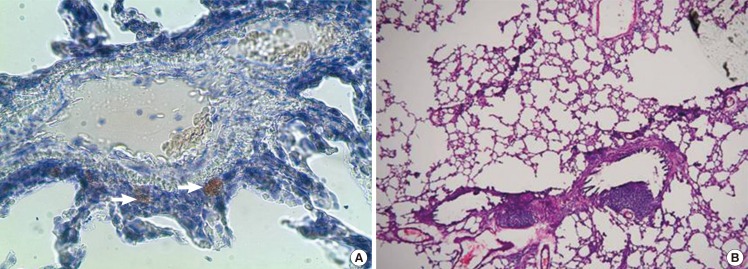
Lung specimen after induced fat embolism
Lung biopsy at 72 hours after injection. (A) Red-colored fat (white arrows) is observed in the alveolar parenchyma (Oil Red O stain, ×200). (B) There are no notable findings such as changes in the vascular structure and acute inflammatory reactions (H&E, ×100).
At 72 hours after infusion in the kidney specimen, there were red-colored fat substances on the Oil Red O stain on the biopsy of the rat kidney in the tissue between the convoluted tubules. In addition, it was confirmed that the intravascular fat droplets permeated the vascular wall and then migrated to the tissue. On the H&E stain, a larger number of foam cells appeared with time (Fig. 6).
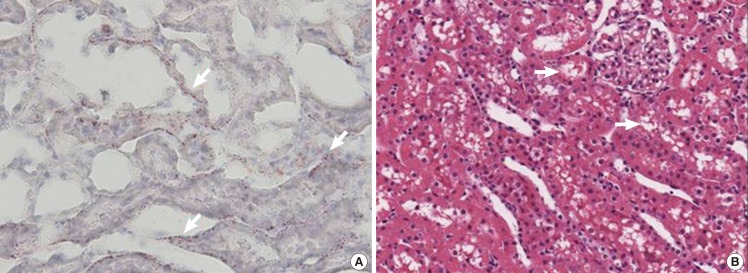
Kidney specimen after induced fat embolism
Kidney biopsy at 72 hours after injection. (A) Red-colored fat (white arrows) is observed in the epithelial cell tissue as compared to that at 24 hours (Oil Red O stain, ×400). (B) Foam cells (white arrows) are observed in the tubular epithelial cells (H&E, ×20)
On the Oil Red O stain on the biopsy of the rat lung, free fat substances were observed in the blood vessels until 24 hours after infusion. At 72 hours after infusion, they were also observed in the extravascular parenchymal tissue. This confirmed that the free fat substances migrated to the extravascular space. On the H&E stain at 24 hours and 72 hours after infusion, however, there were no structural changes or acute inflammatory reactions suggestive of vascular destruction.
On the Oil Red O stain on the biopsy of the rat kidney, free fat substances were observed in the extravascular space at 72 hours, whereas they were presented in the intravascular space at 24 hours after infusion. This confirmed that the free fat substances migrated to the extravascular space. On the H&E stain, the foam cells appeared at each time point. These findings indicate the migration of the fat substance. In addition, an increasing number of foam cells appeared with time.
DISCUSSION
The fat in the human body can be classified into two types: the intravascular transport form of fat and the extravascular storage form of fat. The transport form of fat is present as lipoprotein including chylomicron, very low-density lipoprotein, low-density lipoprotein, and high-density lipoprotein. In addition, the storage form of fat is mostly triacylglycerol and is accumulated in the adipose tissue. The storage form of fat is degraded into free fatty acids when needed. Then, it binds to the serum albumin and can be converted into the transport form of fat [6].
Liposuction is characterized by the physical destruction of the storage form of fat. Therefore, it carries risks of developing severe complications such as fat embolism [7,8]. Although fat embolism is a potentially fatal complication, it usually presents as a subclinical entity. To date, liposuction has been considered a safe procedure [5]. This suggests that the storage form of fat can be removed without causing clinical symptoms after it is released into the intravascular space.
In the human body, the fat is transported in the intravascular space through normal chemical mechanisms in which it binds to the carrier protein. However, little is known about the mechanisms by which the fat substances are transported into the extravascular space without causing clinical symptoms or other problems after they enter the blood vessels through such abnormal processes as liposuction. To clarify these mechanisms, we performed the current experiment by using rats whose anatomical structures, metabolic activity, and fat tissue distribution are similar to those of humans and which are useful for the animal studies [9].
In the current experimental study, the rats did not show any symptoms that are suggestive of embolism. Our results showed that there were no notable structural changes and the intravascular fat was transported into the extravascular space. These results indicate that a massive amount of the intravascular storage form of fat passed the vascular wall without causing any damage and was then transported into the extravascular space. In addition, the epithelial cells lining the convoluted tubules were transformed into foam cells in the rat kidney. This strongly indicates that the epithelial cells might be associated with the fat re-absorption [10].
However, there are certain limitations of the current experimental study; these are as follows: 1) We examined a small number of experimental animals. 2) We monitored our experimental animals for a short period of time (72 hours). 3) The current study is an experiment between different species; human fat oil is infused into the blood vessels of rats in order to induce fat embolism. 4) An accurate amount of fat substance could not be injected into the experimental rats because the fat and water were not homogeneously mixed.
Further studies are warranted to examine the mechanisms by which the storage form of fat can be safely transported into the extravascular space after it has inevitably appeared during liposuction. In the current study, we demonstrated that the intravascular storage form of fat was transported into the extravascular space without causing the clinical symptoms of fat embolism. This indicates that there are also unknown mechanisms by which fat is transported from the intravascular to the extravascular space, which deserve further study. Our results provide a basis for further studies to examine the methods for preventing fat embolism, one of the fatal complications that might occur during liposuction.
Notes
No potential conflict of interest relevant to this article was reported.