Brachial plexus impingement secondary to implantable cardioverter defibrillator: A case report
Article information
Abstract
Overall complication rates of 9.1% have been reported following implantable cardioverter defibrillator (ICD) placement. Brachial plexus injury is infrequently reported in the literature. We describe a 26-year-old female experiencing left arm nerve pain, a positive Tinel’s sign, numbness in the median nerve distribution of the hand and biceps muscle weakness following revision ICD via subclavian vein approach. Nerve conduction studies identified severe partial left brachial plexopathy, which remained incompletely resolved with conservative management. Surgical exploration revealed lateral cord impingement by the ICD generator and a loop of the ICD lead, along with fibrosis, necessitating surgical neurolysis and ICD generator repositioning. As increasing numbers of patients undergo cardiac device implantation, it is incumbent on practitioners to be aware of potential increases in the prevalence of this complication.
INTRODUCTION
Conventional implantable cardioverter defibrillator (ICD) insertion is associated with a reported overall complication rate of up to 9.1% [1]. The device system is placed in either a subcutaneous or subpectoral pocket and consists of one or more leads attached to a generator. Brachial plexus injury following ICD placement is infrequently reported in the literature, where either injury at the time of placement or pacing lead dislodgement have been identified as the aetiological factors [2-5]. Here, we report a case of brachial plexus injury arising from irritation secondary to the pressure from both the ICD generator and the lead.
CASE
A 26-year-old right hand dominant female initially underwent implantation of a single chamber ICD using subclavian vein access in a submuscular pocket following an out-of-hospital cardiac arrest. The generator was placed in a submuscular location due to little subcutaneous tissue in the infraclavicular region. When the battery depleted, she underwent a second procedure under general anesthetic to change the ICD generator (VVI ICD; Medtronic Evera DR pulse generator). This procedure was performed in a different hospital. During the second procedure the initial subpectoral pocket was found to have been placed rather superior and lateral, requiring the formation of an entirely new pocket. The new pocket was lined with an absorbable antibacterial envelope (TYRX). Significant bleeding following removal was controlled using a hemostatic matrix (FloSeal). Wound closure was performed in layers in the usual manner.
The patient reported waking up from the anesthetic with symptoms of left arm nerve pain. Further assessment revealed reduced sensation in the left hand, predominantly the index finger, paresthesia over the lateral aspect of the left forearm, as well as spontaneous twitching of the biceps muscle. Clinical examination showed a positive Tinel’s sign over the ICD generator, weakness of the biceps muscle, reduced sensation over the lateral forearm and within the median nerve distribution. Ulnar and radial nerve function were found to be intact.
Subsequent nerve conduction studies performed within weeks of the ICD placement identified a partial left brachial plexopathy, predominantly affecting the lateral cord segments. An electromyogram not only demonstrated severe denervation of the biceps muscle but also, though less markedly, denervation of the median nerve innervated forearm muscles. Furthermore, evidence of post ganglionic sensory attenuation affecting median and lateral antebrachial nerves was reported.
A Doppler ultrasound scan excluded a subclavian vein thrombus and a computed tomography (CT) angiogram of the neck ruled out a mass lesion as the underlying cause for the brachial plexopathy. Though the CT demonstrated the ICD generator and loop of the ICD lead, it was overall regarded as limited in the evaluation of the brachial plexus involvement (Fig. 1).
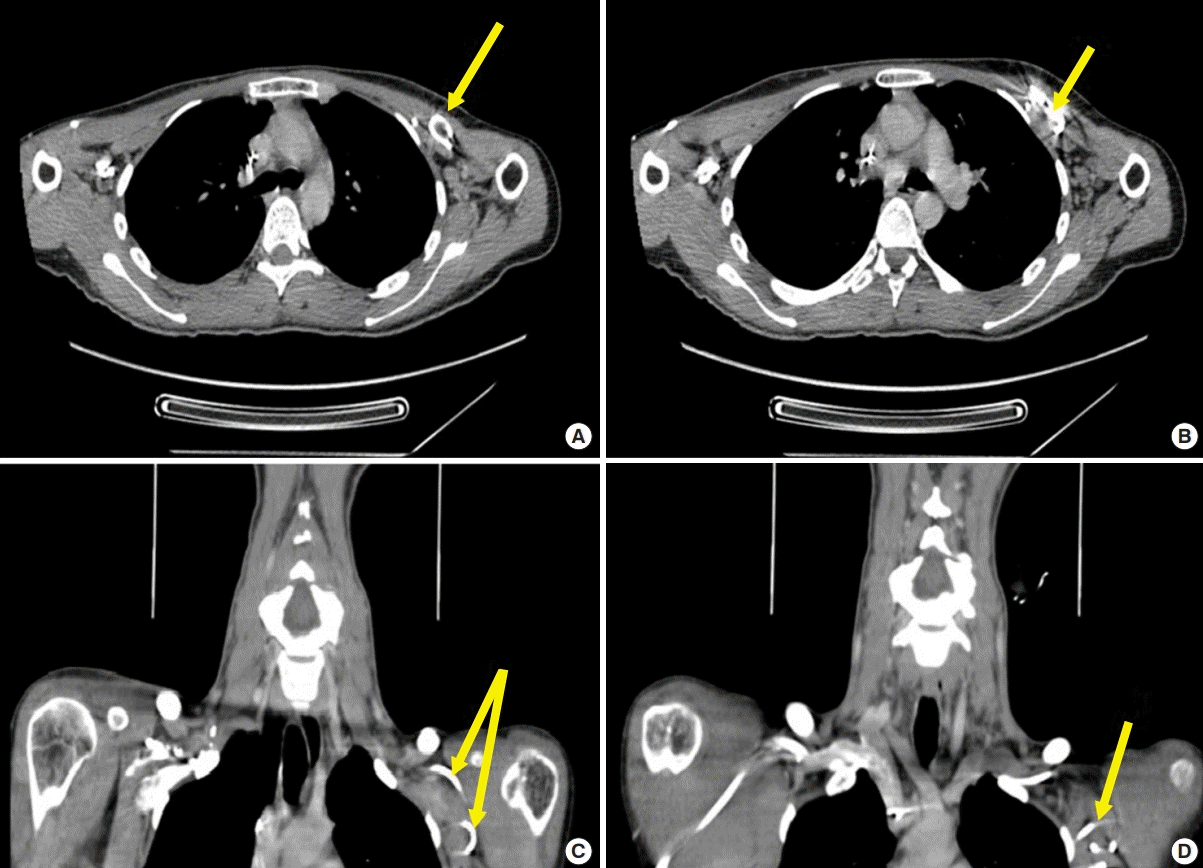
Contrast neck CT images
Axial plane computed tomography (CT) image. (A) Loop of implantable cardioverter defibrillator (ICD) lead identified in left infraclavicular space (yellow arrow). (B) ICD box identified in left infraclavicular space (yellow arrow). (C, D) Coronal plane CT image. Yellow arrows indicate laterally directed loop lead of ICD in left infraclavicular space.
Despite the recovery of the biceps muscle function over the first 4 months and improvement of the neuropathic pain with specialist physiotherapy and pain team intervention, the patient remained unable to fully extend her left shoulder or use her left hand. She continued to have a positive Tinel’s sign and judged the chronicity of her pain as unacceptable.
On this basis, 9 months after generator replacement the patient was referred to the Plastic Surgery Department in University Hospital of South Manchester, where failure to progress over a further 6 weeks resulted in a joint decision by our plastic surgery and cardiology teams to re-explore the infraclavicular plexus to allow for neurolysis and ICD re-positioning as indicated.
Surgical exploration (Fig. 2) revealed that the ICD box and the loop of the ICD lead were pushing upward towards the neck, resulting in compression and chronic irritation of the brachial plexus. Limited neurolysis was performed to release the device from the lateral cord. Repositioning and securing of the ICD generator to a more medial position within the submuscular plane away from the brachial plexus was performed.
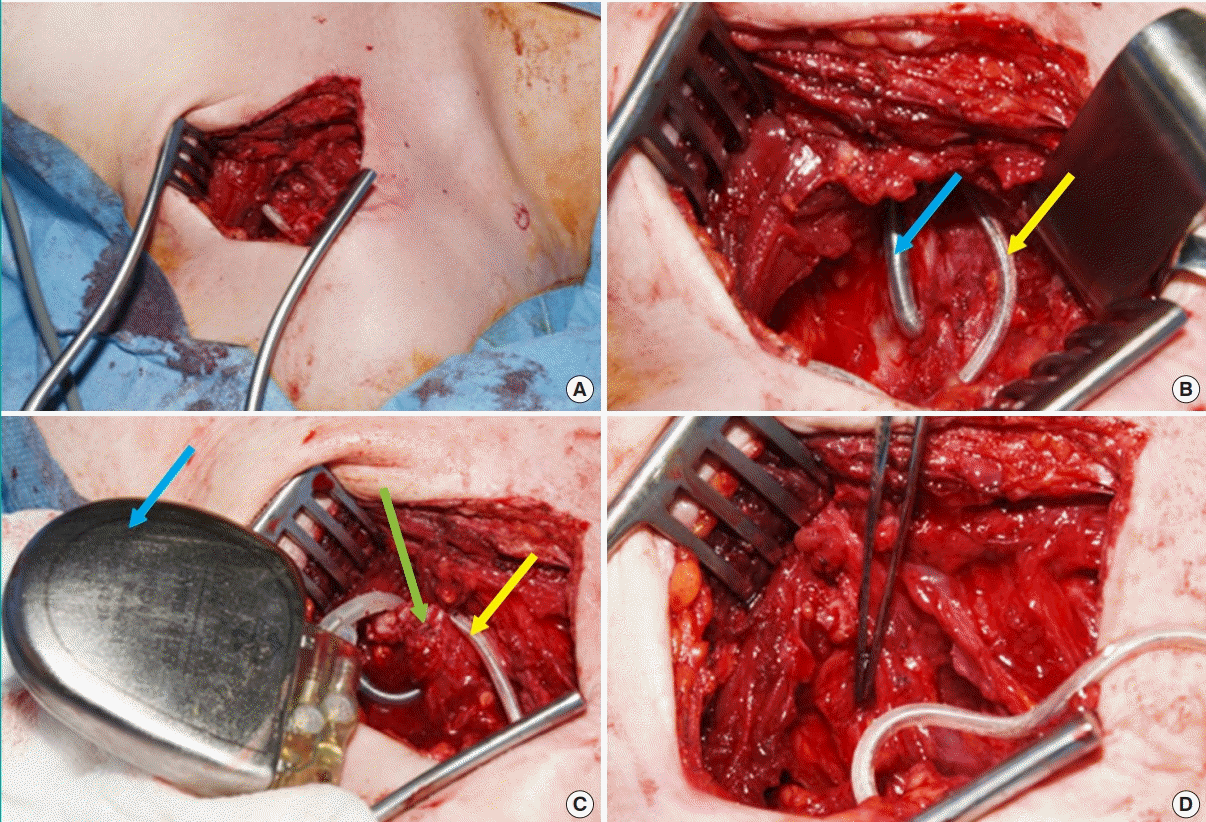
Intraoperative images of ICD repositioning
(A) Access through original left infraclavicular incision. (B) Dissection into subpectoral pocket revealed the implantable cardioverter defibrillator (ICD) lead loop (yellow arrow) from computed tomography and the laterally situated ICD generator (blue arrow). (C) Once the box (blue arrow) was dissected out the loop of ICD lead (yellow arrow) remained tethered by fibrotic scar tissue (green arrow) necessitating neurolysis. (D) Following careful dissection, the loop was freed from the brachial plexus and the box and lead re-positioned. The forceps here is indicating the lateral cord of the brachial plexus.
The postoperative recovery was uneventful and the patient discharged 2 days after surgery. The 6-month follow-up of the patient demonstrated significant improvement in pain and function.
DISCUSSION
Iatrogenic brachial plexus injuries may comprise up to 7%–10% of plexopathies [6]. Neurologic sequelae following ICD insertion may be related to vein puncture site for lead access or generator placement. In the case presented here, it was likely a combination of both factors.
Venous access for lead insertion is traditionally performed using cephalic, axillary or subclavian veins. Intrathoracic subclavian vein approach has been associated with serious complications including lead fracture, subclavian crush syndrome, arterial puncture, pneumothorax and peripheral nerve injury [7]. While early literature indicated a 4.3% incidence of transient brachial plexus injury following subclavian puncture [8], there are relatively few reports in the literature in more recent years. For lead reliability (reduction in the risk of lead entrapment in the costoclavicular ligament) it is desirable to approach the vein as laterally as possible, although this increases the risk of arterial puncture or brachial plexus injury [9]. While reports of brachial plexus injury following axillary, cephalic or internal jugular approach have been described [2,5], the majority of cases are correlated with subclavian puncture. This is thought to be due to brachial plexus compromise resulting from expanding hematoma or direct trauma by the needle [10]. Increasing evidence in the literature promotes axillary over subclavian puncture to avoid crush syndrome or pneumothorax and improve lead longevity [11]. However, both approaches are intimately related to the brachial plexus and therefore compromise of the brachial plexus after damage to either vessel need to be considered. The cephalic cut-down approach is less frequently associated with complications but can only accommodate a single lead and has a tortuous course resulting in more frequent procedural failure [11].
In the case described, the larger subpectoral pocket may have also played a significant role leading to the brachial plexus irritation. Submuscular pockets are currently preferred in the literature over subcutaneous placement of the ICD generator, due to improved cosmesis in patients with little subcutaneous tissue as well as decreased risks of Twiddler’s syndrome and lead dislodgement [9,12]. However, the submuscular pocket is positively associated with a higher bleeding risk, lateral migration of the ICD generator and technical challenges associated with generator replacement [12].
In our case, the initial pocket revision at the time of generator replacement, which caused the brachial plexopathy, aimed to correct the very lateral position of the ICD pocket by creating a new or further submuscular pocket. It may be that subsequently a communication between the two pockets developed, resulting in an overall excessive subpectoral space, allowing for the lateral ICD dislodgement and the impingement on the brachial plexus. It is also noteworthy that significant bleeding occurred during the initial revision replacement. Although no hematoma was identified on imaging, the possibility remains that a hematoma had formed but had been resorbed before the CT scans were performed. The transient compression could still have caused significant nerve injury that persisted after resorption occurred. Similarly, electrocautery used to control bleeding intra-operatively may result in nerve injury that is not recoverable or to prolonged neuropraxia [13].
In summary, our case highlights a number of factors associated with brachial plexopathy during cardiac device implantation and emphasizes the importance of adherence to meticulous technique and awareness of possible anatomical variations, in particular in cases of revision implantation. We advocate a high index of suspicion when confronted with symptoms of brachial plexus injury after cardiac device placements and the value of diagnostic investigations for these cases. Transient brachial plexus blocks following local anesthetic administration during the procedure have been reported [14] but persistent pain and neurological symptoms warrant further investigation and often early surgical exploration to re-position the implant.
As increasing numbers of patients are undergoing cardiac device implantation it is necessary to anticipate complications and be aware of likely increases in the prevalence of associated brachial plexus injury and its management, given the potential long-term disability and variable prognosis [6,15].
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Author contribution
Data collection and literature review: Jumper N, Radotra I. Drafting of manuscript: Jumper N. Critical revision of manuscript for content: Witt P, Mishra A, Campbell NG. Administrative technical and material support: Jumper N, Radotra I, Mishra A. Approval of final manuscript: all authors.
