Clinical outcomes of a low-cost single-channel myoelectric-interface three-dimensional hand prosthesis
Article information
Abstract
Background
Prosthetic hands with a myoelectric interface have recently received interest within the broader category of hand prostheses, but their high cost is a major barrier to use. Modern three-dimensional (3D) printing technology has enabled more widespread development and cost-effectiveness in the field of prostheses. The objective of the present study was to evaluate the clinical impact of a low-cost 3D-printed myoelectric-interface prosthetic hand on patients’ daily life.
Methods
A prospective review of all upper-arm transradial amputation amputees who used 3D-printed myoelectric interface prostheses (Mark V) between January 2016 and August 2017 was conducted. The functional outcomes of prosthesis usage over a 3-month follow-up period were measured using a validated method (Orthotics Prosthetics User Survey–Upper Extremity Functional Status [OPUS-UEFS]). In addition, the correlation between the length of the amputated radius and changes in OPUS-UEFS scores was analyzed.
Results
Ten patients were included in the study. After use of the 3D-printed myoelectric single electromyography channel prosthesis for 3 months, the average OPUS-UEFS score significantly increased from 45.50 to 60.10. The Spearman correlation coefficient (r) of the correlation between radius length and OPUS-UEFS at the 3rd month of prosthetic use was 0.815.
Conclusions
This low-cost 3D-printed myoelectric-interface prosthetic hand with a single reliable myoelectrical signal shows the potential to positively impact amputees’ quality of life through daily usage. The emergence of a low-cost 3D-printed myoelectric prosthesis could lead to new market trends, with such a device gaining popularity via reduced production costs and increased market demand.
INTRODUCTION
Additive manufacturing, especially three-dimensional (3D) printing, is an attractive manufacturing technique that has been recently developed. In 3D printing, products are created layer by layer using a printer, through a process that can easily be customized without changing the equipment. This technique has led to significant developments in various areas, including medicine. In particular, it has facilitated considerable progress in the design and development of medial prostheses. For example, upper-limb prostheses can be effectively designed and printed to fit amputated arms.
With advances in technology and our understanding of the human body, upper-limb prosthetic technology has advanced and become more effective. Nonetheless, the cost of conventionally manufactured prosthetic hands is very high, ranging from $4,000 to $75,000 [1]. The high cost of upper-limb prostheses is a significant barrier to their usage among amputees. To overcome this barrier, 3D-printed prostheses are being made for those who cannot afford an expensive commercial prosthesis. The establishment of e-NABLE has stimulated the worldwide development of 3D-printed prostheses over the last 5 years. Designs of 3D-printed upper-limb prostheses are mostly open-source and freely available on the web. However, no evidence has been reported showing that short- or long-term use of 3D-printed myoelectric interface prostheses has improved the daily life of patients [2]. The objective of the present preliminary study was to evaluate whether 3D-printed upper-limb prostheses could provide higher functionality among transradial amputees, while meeting their financial requirements. The study was designed to evaluate patient-reported outcomes after using the prostheses.
METHODS
Study overview
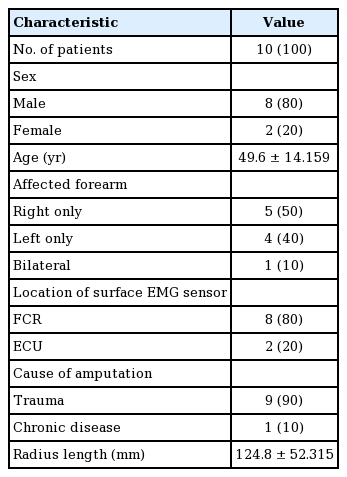
All upper-arm amputees who used 3D-printed hand prostheses between January 2016 and August 2017 were included in this study. The study was approved by the Institutional Review Board of Seoul National University Hospital and performed at the authors’ institution (IK and EJ). All participating patients provided written informed consent and agreed to purchase the 3D-printed prostheses for their daily use. The follow-up period was 3 months. The inclusion criteria for the patients were single-level transradial amputation at or between the radio-carpal and elbow joints. The 3D-printed prosthesis used was a single-channel myoelectric-interface hand prosthesis called the Mark V (Mand.ro, Seoul, Korea), which is commercially available in Korea and costs approximately $1,500 (Fig. 1A). The primarily outcome measure was patient-reported pre-application status and status after 1 month and 3 months of using the 3D-printed manufactured prosthesis. The Orthotics Prosthetics User Survey–Upper Extremity Functional Status (OPUS-UEFS) score was used for the assessment. Other checklists included the time period after amputation surgery, current pain status in the affected limb assessed using a visual analog scale (VAS), and radius length in millimeters assessed using an X-ray image.
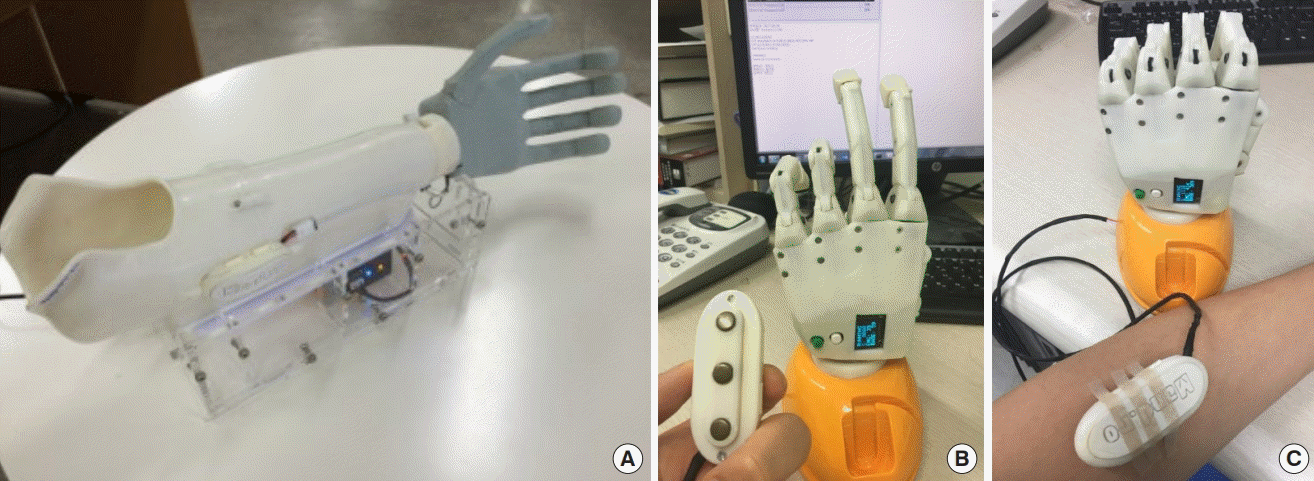
3D printed single channel myoelectric hand prosthesis
(A) The single channel electromyography (EMG) sensor is installed in the socket where the maximal voltage change was check in the amputated forearm. (B, C) The prosthesis model with surface EMG sensor detects the location of the highest voltage change of myoelectrical signal in the stump, when the patient moving the remnant forearm muscle. The voltage change is displayed in the screen on the hand. This model can be used for the pre-application exam and patient’s follow-up. 3D, three-dimensional.
3D-printed myoelectric hand prostheses, the Mark V, and device application
The Mark V is a prosthetic device with surface electromyography (EMG) capability. The device has a small surface EMG sensor that detects myoelectrical signals in the stump (Fig. 1B). The maximal voltage difference when a patient contracts the muscles of the wrist flexors or extensors was used as a reference myoelectric signal to control the device (Fig. 1C). In every patient, the site of the EMG sensor in the support socket showing the maximal voltage difference was identified and used (Fig. 1C). The prostheses were manipulated using the EMG signal produced due to patterns produced throughout the duration of each voluntary muscle contraction (Fig. 2). The grasping function patterns of the device were predefined based on the EMG signal of voluntary muscle contraction (Fig. 3, Supplemental Video 1).
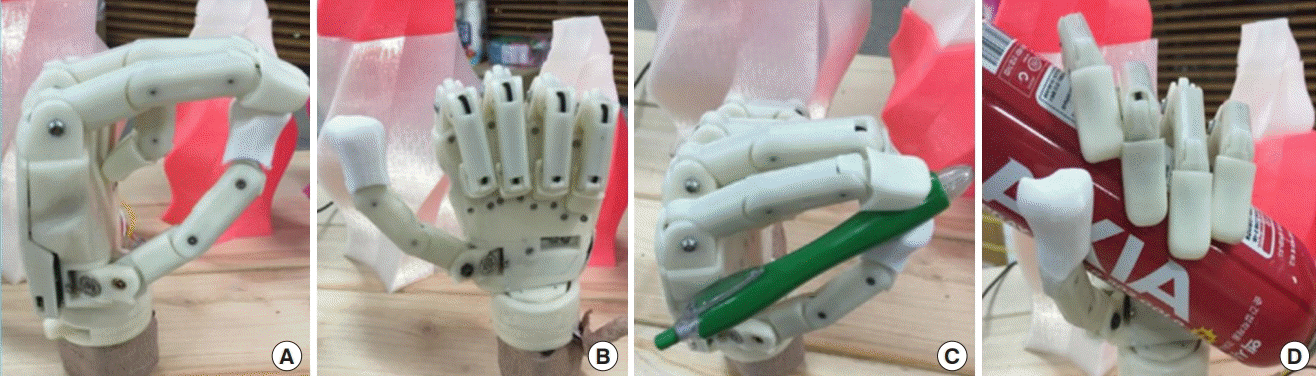
Predefined hand postures
(A-D) The basic postures of hands are programmed in the prosthesis such as pinch, grasping and etc. Photographs by courtesy of Sangho Yi.
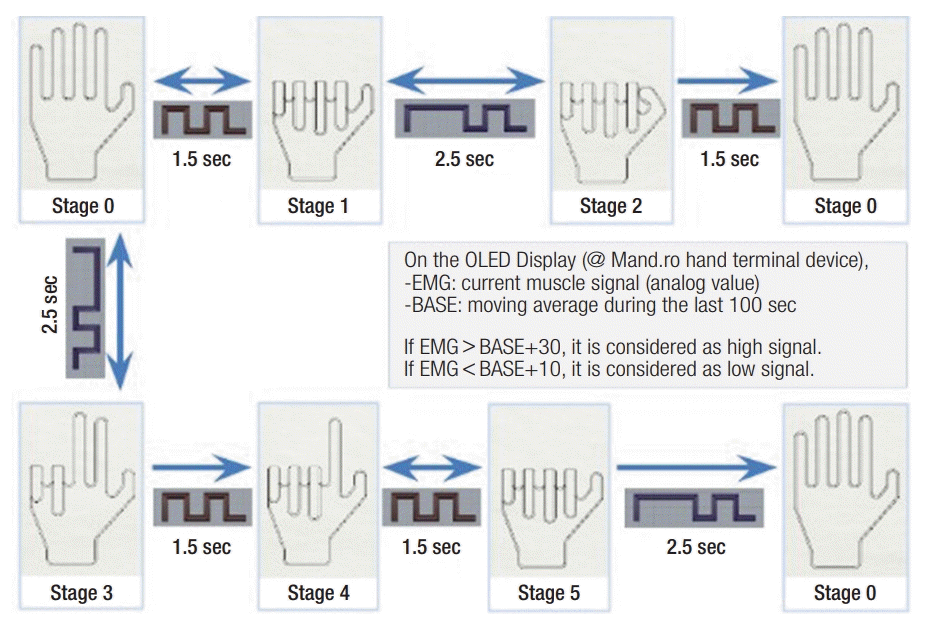
Myoelectrical signals assigned predefine patterns
The upward rectangular curve indicates the duration of muscle contraction and the downward does those of muscle relaxation. Lines and dots indicate the contraction duration of muscle. OLED, organic light-emitting diode; EMG, electromyography. Figure by courtesy of Sanho Yi.
Patient-reported OPUS-UEFS and pain status assessment via VAS
The outcomes after application of the 3D-printed prostheses were assessed by means of patient questionnaires addressing pre-application status and the status at one and three months after prosthetic use. The questions on the OPUS-UEFS addressed 28 activities carried out using the upper extremities to determine how easily the patients could perform them. The scale for each item comprised 6 points, ranging from “very easy” to “not applicable,” and the overall score varied from 0 to 112 (Table 1). The VAS (range, 0–10 points) was used to measure change in pain intensity after application of the device.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The OPUS-UEFS and VAS scores before application of the implant and at 1 and 3 months post-application were compared using the t-test. The threshold for statistical significance was set at P<0.05. The Spearman correlation coefficient (r) was computed to determine the linear associations between the OPUS-UEFS or VAS scores and variables including radius length, age, time period between the last amputation surgery and prosthesis application, and the duration of prosthesis usage.
RESULTS
Sixteen patients enrolled, and six patients were lost during follow-up. Ten patients were included in the final analysis. Eight of the patients were male, and the other two were female. The age of the subjects ranged from 22.7 to 63.6 years. Of the 10 cases reviewed, nine patients were unilateral amputees. Five cases were right forearm amputations, and four cases were left forearm amputations. The nine cases were post-traumatic. The one case involved bilateral forearm amputation due to sequelae of sepsis (Table 1).
Changes in OPUS-UEFS and the VAS scores after application of the prostheses
The analysis of OPUS-UEFS and VAS scores during the study period is shown in Table 2. The statistical analysis showed a gradual increase in OPUS-UEFS scores at 1 and 3 months after application of the prostheses. During the pre-application period, the average OPUS-UEFS score was 45.50, but it increased to 54.00 at 1 month and to 60.10 in the 3rd month of follow-up. The P-values for the differences between OPUS-UEFS scores before prosthesis application and at 1 and 3 months post-application were 0.1063 and 0.0014, respectively. In the 3rd month of prosthesis application, the patients showed significantly better OPUS-UEFS outcomes, improved clinical function, and social adjustment with the prosthesis. The average VAS score for pain decreased from 5.2 to 4.5 at 1 month after the subjects were equipped with the 3D prosthesis and to 4.0 in the 3rd month, with P-values of 0.7 and 0.3, respectively, suggesting the suitability of the 3D-printed prosthesis for long-term usage without discomfort.
Correlation between radius length of the amputees and OPUS-UEFS score
The mean radius length in the 10 patients was 124.8 mm, with a standard deviation of 52.31 mm. The radius length ranged from 50 mm to 234 mm. Patients with a longer radius tended to show a better OPUS-UEFS outcome at each follow-up. Statistically, the radius length and OPUS-UEFS scores showed a high level of agreement (r=0.646, P=0.043 in the 1st month and r=0.815, P=0.004 in the 3rd month). As the subjects became used to the 3D prosthesis, there was a stronger correlation between the radius length of the amputees and their OPUS-UEFS scores. Further, the difference between the OPUS-UEFS after prosthesis application and that before application showed a negative correlation (r=–0.693, P=0.026 in the 1st month and r=–0.634, P=0.049 pre-application). However, the change in the pain score did not show a statistically significant correlation with the change in the OPUS-UEFS score (Table 3).
Correlations between age, time period after amputation, prosthesis usage duration, and OPUS-UEFS score
Other factors, including age, time period after the last amputation surgery, and duration of prosthesis usage per day did not significantly influence the primary outcome score, but the duration of prosthesis usage per day was found to have increased. During the 1st month of use, the average time per day was 4.5 hours, which increased to 5.2 hours per day by the 3rd month (Table 3).
DISCUSSION
In the most recent investigation of limb amputee statistics in the United States, the number of upper-limb amputees was estimated to be 1.2 million in 1996, and the number of individuals undergoing limb amputation each year was estimated to be 185,000 [3]. Although 90% of upper-limb amputations involve previous trauma, the increase in the number of elderly patients and procedures associated with chronic dysvascular disease is expected to double the above estimates by 2050 [4,5]. To meet these demands, prosthesis development using advanced technology to provide more aesthetically and more functionally appealing devices is under active discussion in the industry and among community-level stakeholders.
Recently, large-scale trials have aimed at improving the comfort of amputees, more than a century after the development of prostheses in the 19th century. The early prostheses were not electrically powered, but instead utilized nearby muscles in the ipsilateral shoulder and connected pulling cables to transfer power to the prosthetic joints. This early concept of the body-powered prosthesis has been preserved to the present day. As prosthesis control using electricity and high air pressure became possible, diverse concepts such as the use of transistors to develop externally powered prostheses were attempted during World War II, which was responsible for a surge of upper-limb amputees in the 1950s [6]. The miniaturization of motors and electrical components led to the development of multifunctional myoelectric devices. Improvements in our understanding of neural electrical signals in skeletal muscles aided the development of surface EMG recordings in residual limb muscles to achieve prosthetic control without accessory shoulder movement [7]. More research on targeted muscle reinnervation and direct cerebral cortex stimulation to improve prosthesis control represent a promising future for limb amputees [8,9].
Current commercially available externally powered prostheses for popular usage are based on surface EMG control. In combination with precise, small-sized, low-consumption electromotors and high-speed processing units, the increased functionality of transradial prostheses with appropriate size, low weight, and sufficient power grasp force has been achieved in myoelectric-powered prosthetic hand devices such as the Michelangelo and the BeBionic from Ottobock and the i-limb from Touch Bionics with multiple sensor integration and multi-joint articulation [10]. However, the cost of commercial externally powered prostheses can be as high as $75,000 [11]. These high prices are the main barrier to the usage of these prostheses. Lower socioeconomic status has been found to be an independent predictive factor for an increased risk of limb amputation, and the purchase of prostheses by upper-limb amputees is therefore expected to be highly influenced by their price [12]. Approximately half of the current market of upper-limb prosthetics consists of body-powered prostheses with cable-driven systems [13]. The insufficient functionality of hardware is not the only reason that half of upper-limb amputees choose to purchase body-powered prostheses, although they have a higher rejection rate than externally powered myoelectric prostheses [8].
After the introduction of 3D printing technology, it has been in the spotlight in the manufacturing industry due its potential for cost reduction and customization. The application of 3D printing technology to prosthesis production suggests that it can be an effective lower-cost alternative to pre-existing myoelectric upper-limb prostheses [14]. The manufacturing costs of 51 developed 3D-printed upper-limb prostheses have been estimated to be between $5 to $500 [2]. Although there is a likelihood of higher pricing upon introduction to the market, the price would still be far below that of conventionally manufactured myoelectric prostheses. Moreover, 3D-printed prostheses have other advantages, including freedom of design, personalization and customization capabilities, no assembly requirements, and prompt and inexpensive restoration of impaired prosthesis components. These features have inspired the development of several myoelectrical prostheses with a lower price or using non-profit approaches. The prosthesis used in this study was developed to meet the requirement of lower price and is a popular type of 3D-printed myoelectric hand prosthesis currently available in Korea.
Following the introduction of a new 3D-printed prosthesis, the Southampton Hand Assessment Procedure (SHAP test) is used to evaluate its efficacy [15]. However, the SHAP test does not provide evidence regarding the clinical aspects of user acceptance or functionality over long-term follow-up. Outcome measures for the functional outcomes of upper-limb prostheses have been developed and analyzed. The Assessment of Capacity for Myoelectric Control, Orthotics and Prosthetics Users’ Survey (OPUS), and Trinity Amputation and Prosthesis Experience Scales are three measures used for adult upper-limb amputees [16].
Among these measures, only the OPUS measures clinical performance and emotional function, psychosocial adjustment, body image, and social interaction in addition to adjustment to prostheses [16]. The questions in the OPUS focus on the prosthetist’s care and the involvement of the client in decision-making [17]. The OPUS-UEFS was previously employed to evaluate the effects of the Ottobock Michelangelo prosthesis on activities of daily living (ADLs) [18]. To deepen our understanding of the long-term effects of 3D-printed myoelectric prostheses in upper-limb amputees, we adopted the OPUS-UEFS to assess the potential of a 3D-printed myoelectric prosthesis created with a lower price or a nonprofit goal in mind for enhancing prosthesis dexterity and quality of life. A previous study investigated a 3D-printed body-powered prosthesis and changes in manual dexterity using the box and block test with 2 years of follow-up [19]. However, there is no previous study on a 3D-printed myoelectrical hand prosthesis with a clinical evaluation of patient-reported outcomes.
In our 3-month follow-up study of a low-cost 3D-printed single-myoelectric-interface prosthesis, the OPUS-UEFS score increased significantly by the 3rd month of prosthesis use. The score was not statistically significantly higher at 1 month, because a time-consuming process of adaptation to the new prosthesis is inevitable. Education and rehabilitation programs would aid patients by improving their control.
Among the questions on the OPUS-UEFS, items such as “cutting meat with a knife and fork,” “pouring from a 12 oz can,” “using scissors,” “carrying a laundry basket,” “using a hammer and nail,” “stirring in a bowl,” and “peeling potatoes (or fruit) with a knife/peeler” showed the most outstanding results. Interestingly, these activities all involve hand grasping. The grasping function and basic hand grip concept introduced in the 1950s defines two basic hand grasps, known as the power grip and precision grip [20]. A power grip consists of a prehensile movement in which an object is grasped by the fingers and pressed against the buttress of the thumb and its intrinsic muscles, while a precision grip is an accurate prehensile action in which an object may be held away from the palm between the thumb and fingertips [21]. Compared with high-end commercial multiple-channel myoelectric hand prostheses such as the Ottobock Michelangelo, which can facilitate power grip, precision grip, and other hooking, tripod, spherical, and lateral grip patterns of the hand to perform ADLs, the Mand.ro Mark V is capable of performing the power grip and several predefined grips with just a single EMG channel. Although it is difficult to draw precise parallels between the OPUS-UEFS outcomes for the Ottobock Michelangelo in the previous report and the Mand.ro Mark V, as the scales of measure, follow-up period, and number of subjects were different, the use of a 3D-printed prosthesis clearly increased the OPUS-UEFS score [22]. Among physicians, the low functionality of the traditional myoelectric prosthesis is the major barrier to their widespread utilization in clinical practice. In the present study, we found that focusing on the grasping function, instead of complicated functions, enabled patients to perform ADLs more easily.
Currently, myoelectric 3D-printed hand prostheses mostly provide five fingers. This appearance transcends the traditional prosthesis from an aesthetic perspective. With five fingers, myoelectric 3D-printed prostheses such as the Dextrus EMG, HACKberry, and Handiii provide precision grip function and four other types of grips, similar to the Ottobock Michelangelo [2]. This advancement in 3D-printed prostheses has narrowed the differences in clinical outcomes between the Ottobock Michelangelo and 3D-printed prostheses, and is promising in terms of the future popularity of 3D-printed myoelectric prostheses.
In the present study, patients with a longer radius showed better OPUS-UEFS scores at each follow-up. When the radius length is preserved, more preservation of functional muscles is expected, which would be beneficial in producing effective signals for the prosthesis. In addition, preservation of the radius length, which results in a longer lever arm, allows for the generation of greater torque, improving the functional utility of the prosthesis [23]. This suggests that for amputees planning to wear a functional prosthesis, extensive limb shortening with the bones and muscles should be avoided. In this study, patients’ average VAS score also decreased after the subjects were equipped with the 3D-printed prostheses at each follow-up. Though the result was not statistically significant, this suggests that patients did not actually feel increased discomfort caused by the usage of 3D-printed prostheses, suggesting the suitability of the 3D-printed prosthesis for long-term usage. However, further investigation is required.
To improve the ease of ADLs and to reduce differences between normal hand function and prosthetic function, researchers are seeking ways to provide perfect sensations to the prosthesis [24]. Alternatively, other than applying a prosthesis, hand transplantations have also achieved functional and aesthetic success. However, there are still limitations regarding the use of this approach and the immunological issues involved. In this context, 3D-printed prostheses are gaining popularity. The main advantage of 3D-printed myoelectric prostheses is their low manufacturing costs, far below those of existing myoelectric prostheses; however, no studies have been carried out to predict their life cycle, and studies on their strength and durability are needed [2].
In addition to the above considerations, the limitations of the present study are that it was not a randomized control study, the patients did not receive occupational training, and only self-reported outcome measures were used. Nonetheless, the low-cost 3D-printed myoelectric interface prosthetic hand may have significant potential to positively impact quality of life through daily usage. We observed that even without multiple channels, a single reliable myoelectrical signal was useful and allowed patients to sufficiently modulate the frequency of muscle contraction.
Notes
The Mark V hand prosthesis is a commercial product of Mand.ro in Seoul, Korea. None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Ethical approval
The study was approved by the Institutional Review Board of SMG-SNU Boramae Medical Center (IRB No. 26-2016-99) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Author contribution
Conceptualization: Jeong E. Data curation: Jeong E. Formal analysis: Jeong E. Funding acquisition: Jeong E, Park CY. Methodology: Jeong E. Project administration: Jeong E, Park CY, Lee J. Visualization: Jeong E, Lee GK. Writing - original draft: Ku I, Jeong E. Writing - review & editing: Lee GK. Approval of the final manuscript: all authors.
Supplementary Material
Supplemental Video 1. The grasping function for holding a bottle. Video by courtegy of Sangho Yi.
Supplemental data can be found at: https://doi.org/10.5999/aps.2018.01375.v001