Surgical outcomes of sternal rigid plate fixation from 2005 to 2016 using the American College of Surgeons-National Surgical Quality Improvement Program database
Article information
Abstract
Background
Sternal rigid plate fixation (RPF) has been adopted in recent years in high-risk cases to reduce complications associated with steel wire cerclage, the traditional approach to sternal closure. While sternal RPF has been associated with lower complication rates than wire cerclage, it has its own complication profile that requires evaluation, necessitating a critical examination from a national perspective. This study will report the outcomes and associated risk factors of sternal RPF using a national database.
Methods
Patients undergoing sternal RPF from 2005 to 2016 in the American College of Surgeons-National Surgical Quality Improvement Program were identified. Demographics, perioperative information, and complication rates were reviewed. Logistic regression analysis was performed to identify risk factors for postoperative complications.
Results
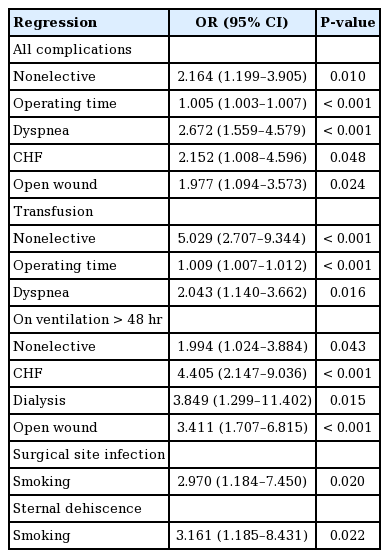
There were 381 patient cases of RPF identified. The most common complications included bleeding (28.9%), mechanical ventilation >48 hours (16.5%), and reoperation/readmission (15.2%). Top risk factors for complications included dyspnea (odds ratio [OR], 2.672; P<0.001), nonelective procedure (OR, 2.164; P=0.010), congestive heart failure (OR, 2.152; P=0.048), open wound (OR, 1.977; P=0.024), and operating time (OR, 1.005; P<0.001).
Conclusions
Sternal RPF is associated with increased rates of three primary complications: blood loss requiring transfusion, ventilation >48 hours, and reoperation/readmission, each of which affected over 15% of the study population. Smokers remain at an increased risk for surgical site infection and sternal dehiscence despite RPF’s purported benefit to minimize these outcomes. Complications of primary versus delayed sternal RPF are roughly equivalent, but individual patients may perform better with one versus the other based on identified risk factors.
INTRODUCTION
Optimization of sternal closure following trauma or median sternotomy is of great interest to multiple surgical specialties due to frequent complications including postoperative mediastinitis, sternal nonunion, or sternal malunion in high-risk cases [1-3]. Postoperative mediastinitis is one of the most lethal complications, with reported mortality rates varying between 10% and 47%, and often requires extensive debridement and reconstruction for treatment [2,4]. Sternal nonunion and malunion are associated with poor outcomes including sternal instability and high rates of postoperative pain. Furthermore, these factors can modify the existing biomechanics of the thoracic cage, compromising regular anatomic ventilation [1,2,5]. These complications underscore the importance of appropriate wound closure for both prevention and treatment.
The traditional approach to sternal closure utilizes steel wire cerclage. This method is associated with complications including bony nonunion and infection, especially in high-risk patient populations. High-risk patients are defined as those with three or more of the following comorbidities: obesity (body mass index >30 kg/m2), diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic steroid use, concurrent infection and acquired or iatrogenic immunosuppression [3,4]. Rigid plate fixation (RPF) in many parts of the body has long been favored by neurosurgery, hand, oral and maxillofacial, and orthopedic surgery over traditional wire cerclage given improved stability and a lower incidence of nonunion, malunion, and infection, yet has not become standard of care in other surgical specialties [3,6]. A number of cadaveric models assessing the biomechanics of wire cerclage and sternal RPF report the advantages of the latter [7]. Clinical outcomes of plating also show advantages over wire cerclage. In two randomized clinical trials, sternal RPF in high-risk cardiac patients resulted in improved wound healing, fewer complications, and decreased postoperative pain [6,8]. The literature also supports a clear benefit of sternal RPF in cases where traditional sternal repair with wire cerclage was initially attempted, and failed [5,9,10]. Vos et al. [9] explain that when wire cerclage fails, the sternum’s structural integrity is destroyed, and subsequent reconstruction with cerclage is almost always impossible. Therefore, a RPF would be advantageous due to its ability to provide structural stability and would potentially obviate the need to solely undergo soft tissue reconstruction [9]. Another potential explanation is that initial placement of wire cerclage can disrupt sternal vasculature and thereby cause malperfusion complications including delayed wound healing, infection, and dehiscence [7]. Such sequelae are less likely to occur in plating due to its non-circumferential contact with the sternum [7]. These documented benefits could explain the recent adaptation of and increased usage of sternal RPF across surgical specialties. However, further study of this technique is needed to improve patient selection and guide its appropriate application. A descriptive analysis of this procedure using a large national database would facilitate the evaluation of patients most likely to benefit from this procedure while also allowing for identification of risk factors associated with adverse outcomes.
This study aims to identify the patient characteristics and risk factors for complications associated with sternal RPF and describe surgical outcomes between sternal RPF performed in a delayed versus primary procedure.
METHODS
Patient selection
The American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) is a prospective, risk-adjusted registry that includes demographic, perioperative, and 30-day postoperative de-identified patient information (https://www.facs.org/quality-programs/acs-nsqip). Patients that underwent sternal RPF were identified using ACS-NSQIP database for the years 2005 to 2016 using Current Procedure Terminology (CPT) codes of 21,750 (closure of median sternotomy separation with or without debridement) and 21,825 (open treatment of sternum fracture with or without skeletal fixation). Only patients with missing data were excluded. Patient demographics, perioperative information, and postoperative outcomes were retrieved. Delayed procedures were classified based on principal procedure (principle CPT) codes and primary based on concurrent and other procedure (concurrent and other CPT) codes.
Patient demographics, clinical factors, and medical comorbidities were compared between patients with and without complications. Additionally, patients undergoing delayed RPF during the index procedure were analyzed and compared to those undergoing RPF as a separate, independent procedure at time of closure. Plating outcomes for each surgical subspecialty were measured using the principle and concurrent CPT codes.
Postoperative primary outcomes evaluated included sternal dehiscence and surgical site infection (SSI). SSI can be further subcategorized as per the CDC surgical wound classifications, from clean (class I) to dirty-infected (class IV). Secondary outcomes included sepsis/septic shock, urinary tract infection (UTI), blood loss requiring transfusion, deep vein thrombosis (DVT), pulmonary embolism (PE), flap or graft failure, and unplanned reoperation/readmission related to primary procedure. Reoperation/readmission was based off the built-in NSQIP variables for reason of reoperation/readmission, as defined by ICD-9 codes. SSI was further subcategorized into: superficial SSI, deep SSI, and organ space SSI. Flap failure was determined using the ACS-NSQIP specific variable for years 2010 and prior. After 2010, reported inaccuracies in the ACS-NSQIP data in relation to this variable required us to use ICD-9 codes 996.52 (mechanical complication due to graft of other tissue, not elsewhere classified), 996.74 (other complications due to other vascular device, implant, and graft), and 996.79 (other complications due to other internal prosthetic device, implant, and graft) [9,11]. A detailed list of patient characteristics can be found in Table 1.
Statistical analysis
All analyses were performed using IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA). The two-sided Student t-test or Welch t-test (to control for sample size difference) were used to assess the differences of the means for continuous variables. Continuous variables are reported as mean and standard deviation (SD). Pearson chi-square test was performed for categorical variables and was used to determine the differences of proportions between groups. Categorical variables are represented as frequencies with corresponding percentages. Variables with an unadjusted P-value <0.25 from chi square analyses were entered into a multivariate logistic regression model to identify independent risk factors for complications. Odds ratios (OR) and 95% confidence intervals (CI) for each independent risk factor were derived. All tests were two-tailed, and a value of P<0.05 was considered statistically significant.
RESULTS
Overall patient characteristics and complication profile
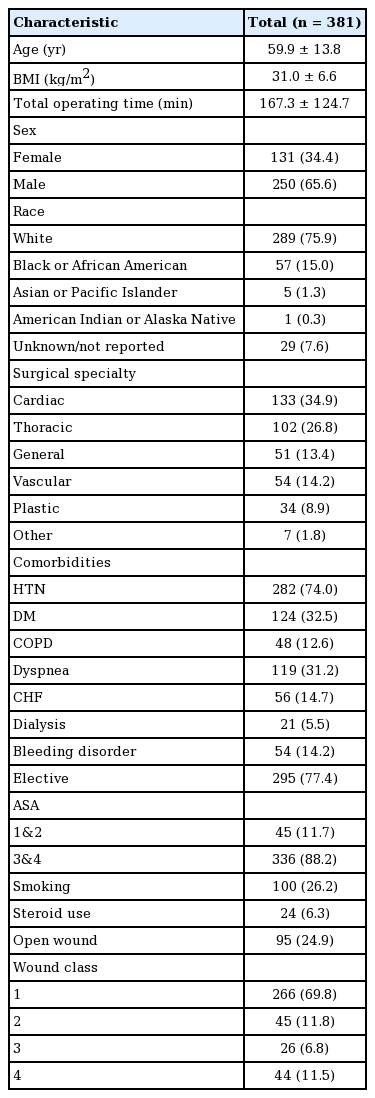
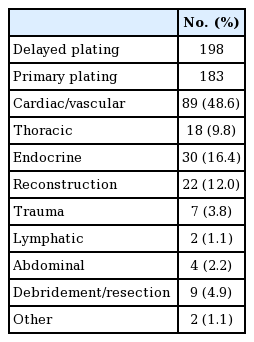
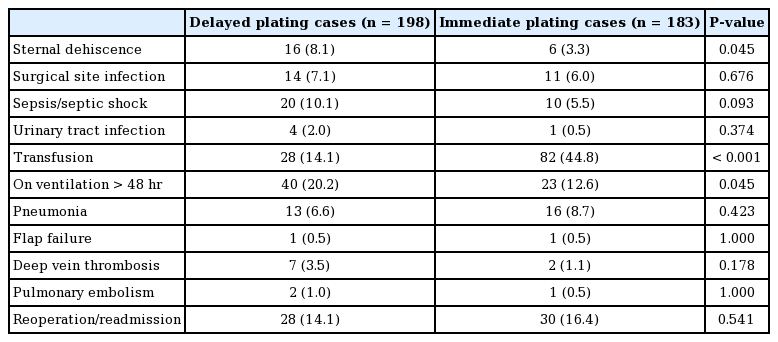
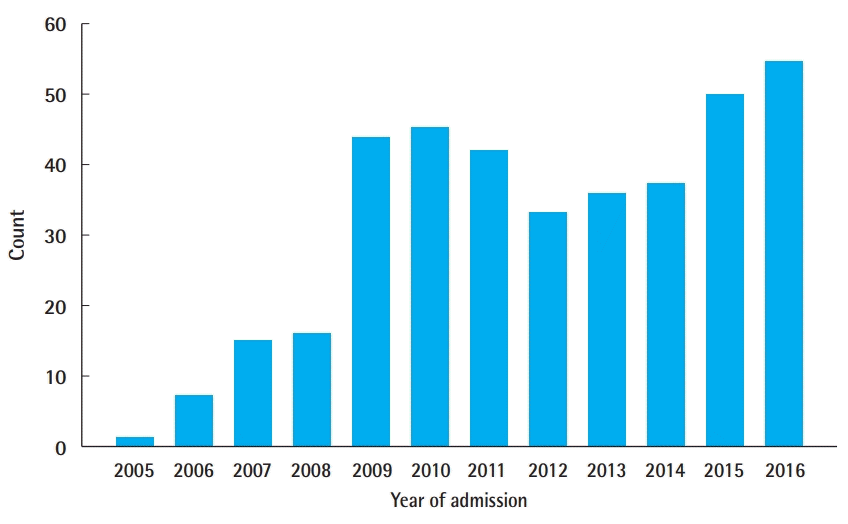
Between 2005 and 2016, 381 cases of sternal RPF were identified using the ACS-NSQIP database. The majority of cases (81%) occurred after 2008 (Fig. 1). Of these, 198 plating cases (52%) were classified as delayed and 183 (48%) were performed during the time of other procedures. The most common surgical services in which patients received primary sternal RPF were, in descending order, cardiac/vascular (48.6%), endocrine (16.4%), reconstruction (12.0%), thoracic (9.8%), debridement/resection (4.9%), trauma (3.8%), abdominal (2.2%), lymphatic (1.1%), and other (1.1%) (Table 2).
Sternal rigid plating from 2005 to 2016
Number of sternal rigid plate fixation cases from 2005 to 2016 using American College of Surgeons-National Surgical Quality Improvement Program.
Mean patient age was 59.9 (SD 13.8). The majority of patients were male (65.6%), white (75.9%), and had an American Society of Anesthesiologists (ASA) physical status classification of 3 and 4 (88.2%). Patients had a high index of comorbidities, including obesity (mean body mass index, 31.0 kg/m2; SD, 6.6), hypertension (74.0%), diabetes mellitus (32.5%), dyspnea (31.2%), congestive heart failure (CHF; 14.7%), bleeding diathesis (14.2%), COPD (12.6%), and chronic kidney disease (CKD; 5.5%) (Table 1).
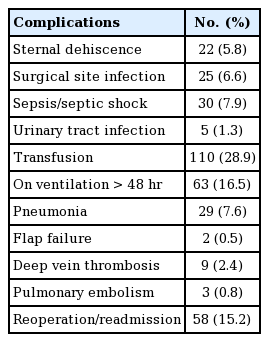
Complications in order of decreasing incidence included: blood loss requiring transfusion (28.9%), mechanical ventilation >48 hours (16.5%), reoperation/readmission (15.2%), sepsis/septic shock (7.9%), pneumonia (7.6%), sternal dehiscence (5.8%), DVT (2.4%), UTI (1.3%), PE (0.6%), and flap failure (0.5%) (Table 3).
Subgroup analysis
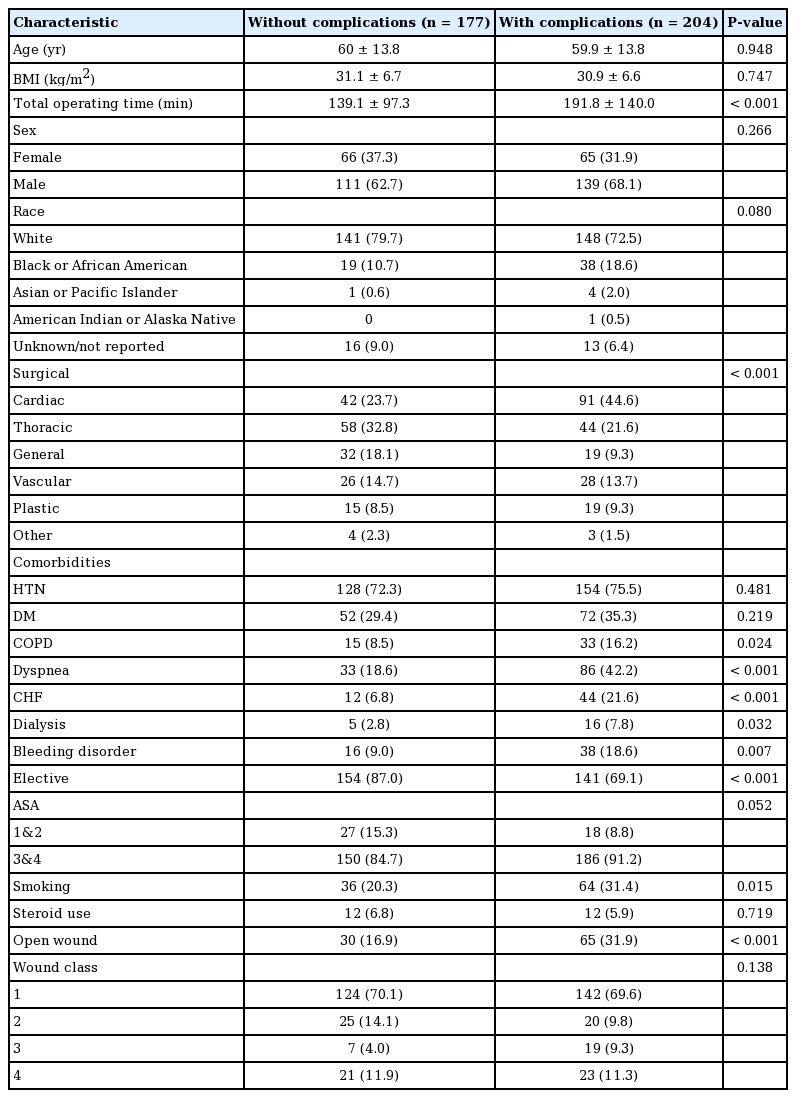
Patients with complications versus no complications had longer total operating time (192 minutes vs. 139 minutes; P<0.001) and higher incidence of existing comorbidities (COPD: 16.2% vs. 8.5%, P=0.024; dyspnea: 42.2% vs. 18.6%, P<0.001; CHF: 21.6% vs. 6.8%, P<0.001; CKD: 7.8% vs. 2.8%, P=0.032; bleeding disorder: 18.6% vs. 9.0%, P=0.007; smoking: 31.4% vs. 20.3%, P=0.015; open wound: 31.9% vs. 16.9%, P<0.001). They had a lower incidence of elective surgery (69.1% vs. 87.0%; P<0.001) (Table 4).
Regression analysis identified dyspnea (OR, 2.672; P<0.001), nonelective cases (OR, 2.164; P=0.010), CHF (OR, 2.152; P=0.048), open wound (OR, 1.977; P=0.024), and increased operating time (OR, 1.005; P<0.001) as independent risk factors for overall complications. Similar analysis was performed for complications of transfusion, mechanical ventilation greater >48 hours, SSI, and sternal dehiscence. Nonelective cases (OR, 5.029; P<0.001), dyspnea (OR, 2.043; P=0.016), and increased operating time (OR, 1.009; P<0.001) were independent risk factors for transfusion. CHF (OR, 4.405; P<0.001), dialysis (OR, 3.849; P=0.015), open wound (OR, 3.411; P<0.001) and nonelective cases (OR, 1.994; P=0.043) were risk factors for ventilation >48 hours. Smoking was an independent risk factor for sternal dehiscence (OR, 3.161; P=0.022) and SSI (OR, 2.970; P=0.020) (Table 5).
Patients who underwent delayed RPF had a higher incidence of sternal dehiscence (8.1% vs. 3.3%; P=0.045) and mechanical ventilation greater than 48 hours (20.2% vs. 12.6%; P=0.045), and a lower incidence of transfusion (14.1% vs. 44.8%; P< 0.001) (Table 6).
DISCUSSION
RPF has traditionally been used as standard of care in some surgical subspecialties. Studies documenting its association with reduced postoperative morbidity, decreased pain, and lower complication profile are likely responsible for its increased use across specialties for sternal closure [3,6,8,9,12]. We identified an uptick in sternal RPF beginning in the year 2008 for both primary and delayed procedures. In the setting of increased usage, our findings emphasize the need to carefully examine procedures with optimal surgical outcomes and identify patients that will best benefit from this procedure modality.
Three complications (blood loss requiring transfusion, ventilation >48 hours, reoperation/readmission) occurred 15% in the overall cohort. Of these, transfusion was required in over a quarter of patients (28.9%). Findings from a previous NSQIP study revealed that the percent of patients requiring intraoperative transfusion who died or developed serious morbidity was 11.3% and 55.4%, respectively, compared with 1.3% and 6.1% in non-transfused patients (both P<0.001) [13]. These complications are not trivial nor infrequent, and patients at increased risk for developing them should be properly informed about their increased risk, while physicians and patients should work together to minimize risk factors as much as possible preoperatively. While these are all complications not exclusive to RPF, the rate of complication and patient characteristics most relevant to developing them are of great importance.
Patients who developed a complication within the 30-day period that is reported by NSQIP were more likely to have the following comorbidities: smoking, COPD, CHF, dyspnea, bleeding disorders, and CKD on dialysis. Overall, this represents a high-risk patient cohort with multiple comorbidities, including cardiovascular disease. Studies by Gummert et al. [4] and Song et al. [3] report that cardiac or high-risk patients undergoing steel wire cerclage tend to have worse outcomes. Although sternal RPF overall tends to have lower complication rates than wire cerclage, the higher complication rate found in this patient cohort can likely be explained by the heightened percentage of patients with multiple comorbidities. However, this cannot be interpreted to mean that complications are unavoidable. In fact, these findings highlight the need for improved patient education regarding procedure risks and outcomes based on their own comorbidity profile. Furthermore, 77.4% of cases were performed on an elective basis. This emphasizes the need to optimize patients preoperatively, whenever possible, before initiating surgical workup in order to help improve surgical outcomes.
Patients who developed complications also had more preoperative open wounds. These potentially non-sterile or complex wounds could predispose patients to an increased risk of postoperative adverse outcomes. As open wounds are managed in a variety of settings, this provides an opportunity for a multidisciplinary approach to work toward optimal wound healing before sternal reconstruction. Primary care physicians, reconstructive surgeons, and the surgeon involved in the original sternal procedure can all contribute to a well-monitored, clean wound preoperatively. In an acute, traumatic setting, these considerations would be less relevant. In fact, our study confirmed that nonelective procedures were associated with an increased risk of complications. Finally, patients who developed complications had a longer average operating time, which has been associated with higher complication rates, potentially due to anesthesia time, complexity of the procedure, or intraoperative adverse events [14,15]. The majority of the patient cases identified in this study (88.2%) were ASA class 3 and 4, suggesting that they were likely not ideal candidates for anesthesia, which could explain the lengthened operating and anesthesia time. If a patient is deemed hemodynamically stable, surgeons could consider temporary sternal closure, with a delayed sternal RPF procedure, to potentially mitigate this risk.
The independent risk factors evaluated included procedure status (elective/nonelective), CHF, presence of open wound, total operating time, dialysis, and dyspnea. A nonelective procedure was a risk factor for transfusion requirement and mechanical ventilation >48 hours, likely again because patients who require emergent operation are acutely more critical patients who would inherently be at risk for complications. Studies across various surgical disciplines have repeatedly found that emergent procedures are independent predictors of morbidity and mortality [16,17]. An open wound site was a risk factor for requiring mechanical ventilation >48 hours, which could be explained by the need to perform serial debridement and washout could be performed before plating [18]. Additionally, these patients may have remained intubated during these sequential operations.
Smoking
Smoking was the only independent risk factor for SSI and sternal dehiscence. While this is commensurate with previous findings addressing wound-based complications, it is still notable, as sternal RPF has been associated with a lower risk of infection and dehiscence overall [19,20]. It is important for both treating physicians and patients who are active smokers to be aware that this technique may not be as effective for them in preventing these complications over wire cerclage, reinforcing the need for preoperative smoking cessation and/or alternative closure techniques in this group.
Delayed versus primary plating
We assessed surgical cases in which sternal plating was performed as a delayed, subsequent procedure versus immediately during a primary operation involving the sternum. A delayed plating closure approach may be utilized to address postoperative complications, such as sternal wound dehiscence or breakdown requiring a reoperation. An increased risk of dehiscence may be explained by the fact that fixation as a delayed, secondary operation would not be performed on a healthy sternum, but rather one that has dehisced and is already susceptible to further complications. Reconstructive surgeons should consider an additional temporal delay to decrease the risk of postoperative infection, with emphasis on the adaptation and implementation of optimal preoperative wound care techniques. There is also potential to reduce this complication if patients who are at highest risk for sternal dehiscence are identified prior to their primary procedure. This would allow for primary plating to be pursued instead.
Patients who underwent delayed plating were at decreased risk of requiring a transfusion. Based on the relatively equivalent complication profile associated with undergoing delayed sternal RPF in comparison to primary, and previously reported benefits of this procedure over wire cerclage, reconstructive surgeons may reduce poor outcomes by pursuing RPF in patients who present with postoperative complications from steel wire cerclage or other sternal closure complications. Although only 8.9% of sternal RPF in our study was performed by plastic surgeons, this procedure is likely to gain more acceptance in this subspecialty as its application for sternal procedures, including reconstruction after invasive sternal cancer or reoperation and surgical closure requiring plastic surgery services [2,5,12,21]. Future studies are needed to investigate whether there are different trends over time in delayed sternal RPF based on index procedure, as well as the distinct complication and risk factor profiles associated with each.
Cost
It is important to consider the economic burden of the marked increase in utilization of this technique. In a randomized trial, Allen et al. [22] found that patients undergoing sternotomy who were closed with RPF had better sternal healing confirmed with imaging, fewer complications, and no additional costs when compared with patients who were closed using wire cerclage at 6 months after surgery. Although the initial procedure and hospitalization was about $2,800 higher per patient undergoing RPF when compared with wire cerclage, costs of complications were $2,297 lower per patient, readmissions $1,462 lower per patient, and outpatient costs were $992 lower per patient [22]. Therefore, the upward trend in utilization we found in this study is supported by a sustainable economic analysis that suggests increased utilization of this procedure will reduce costs in the long term.
Limitations
We acknowledge the limitations of our study. In utilization of the ACS-NSQIP, we are limited by the variables and procedures provided in the database, as well as potential errors in the data collection and distribution process. Some notable specific complications that we could not assess include postoperative mediastinitis, and sternal malunion or nonunion, as ACS-NSQIP is more apt to report more generalizable surgical complications. In addition, we are unable to comment on size or depth of defects, nor specific details of patient presentation severity outside the confines of ACS-NSQIP. These patient characteristics may include cross clamp time, cardiopulmonary bypass time, ejection fraction, and overall diabetes severity. We were also not able to assess outcomes that occurred after the 30-day follow up period, which may result in under-reporting of some complications. Complications on which we were not able to comment include most notably chronic pain and painful palpable hardware. Despite these limitations, our study, to the best of our knowledge, is the first such national database study assessing the patient characteristics and overall scope of complications and risk factors for procedures involving sternal plate fixation.
Conclusion
While the indications for sternal RPF are still being developed in the high-risk population, our study found high rates of three significant complications: transfusion, mechanical ventilation >48 hours, and reoperation/readmission, each of which affected over 15% of the total patient population studied. Smokers are still at risk for SSI and sternal dehiscence despite RPF being proposed to minimize these adverse outcomes. Surgeons should feel comfortable pursuing sternal RPF in a delayed or primary setting, as the complication profiles are roughly equivalent.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
Deidentified patient information is freely available to all institutional members who comply with the ACS-NSQIP Data Use Agreement. The Data Use Agreement implements the protections afforded by the Health Insurance Portability and Accountability Act of 1996 and the ACS-NSQIP Hospital Participation Agreement.
Author contribution
Conceptualization: Tran BN, Fukudome EY, Lee BT. Data curation: all authors. Formal analysis: all authors. Project administration: all authors. Visualization: all authors. Writing - original draft: all authors. Writing - review & editing: all authors. Approval of final manuscript: all authors.