Subtotal calvarial vault reconstruction utilizing a customized polyetheretherketone (PEEK) implant with chimeric microvascular soft tissue coverage in a patient with syndrome of the trephined: A case report
Article information
Abstract
The syndrome of the trephined is a neurologic phenomenon that manifests as sudden decline in cognition, behavior, and sensorimotor function due to loss of intracranial domain. This scenario typically occurs in the setting of large craniectomy defects, resulting from trauma, infection, and/or oncologic extirpation. Cranioplasty has been shown to reverse these symptoms by normalizing cerebral hemodynamics and metabolism. However, successful reconstruction may be difficult in patients with complex and/or hostile calvarial defects. We present the case of a 48-year-old male with a large cranial bone defect, who failed autologous cranioplasty secondary to infection, and developed rapid neurologic deterioration leading to a near-vegetative state. Following debridement and antibiotic therapy, delayed cranioplasty was accomplished using a polyetheretherketone (PEEK) implant with free chimeric latissimus dorsi/serratus anterior myocutaneous flap transfer for vascularized resurfacing. Significant improvements in cognition and motor skill were noted in the early postoperative period. At 6-month follow-up, the patient had regained the ability to speak, ambulate and self-feed—correlating with evidence of cerebral/ventricular re-expansion on computed tomography. Based on our findings, we advocate delayed alloplastic implantation with total vascularized soft tissue coverage as a viable alternative for reconstructing extensive, hostile calvarial defects in patients with the syndrome of the trephined.
INTRODUCTION
The syndrome of the trephined (SoT), or sinking skin flap syndrome, is defined as cognitive and neurologic dysfunction due to an acquired skull defect with reversibility of symptoms after cranial reconstruction [1,2]. The condition affects up to 24% of patients who have undergone decompressive craniectomy [2]. The risk for neurologic decline increases with the size of the craniectomy, which is typically performed in the setting of neurotrauma, hemorrhage, ischemia, infection, or oncologic ablation [2]. Symptoms include weakness, sensory deficits, gait disturbances, impaired cognition, and slowed speech [2]. Onset of symptoms may be acute or chronic, but the average time to presentation is 5 months after craniectomy [1]. The pathophysiology of this syndrome is theorized to begin with the loss of intracranial domain, which permits atmospheric pressure to compress the brain, thereby restricting cerebral perfusion and cerebrospinal fluid (CSF) flow [1-3]. The change in hydrodynamics subsequently alters metabolic pathways, contributing to cortical dysfunction over time [2].
Cranioplasty with re-establishment of intracranial domain is currently the first-line treatment for patients with SoT, and early surgical intervention is associated with improved functional outcomes [2]. The basic goals of calvarial reconstruction include cerebral protection, cosmesis, and normalization of cerebral hydrodynamics and metabolism [4,5]. However, in cases of complex cranial defects with a history of infection, radiation, and/or prior surgery, key decisions must be made regarding the choice of reconstructive material, type of soft tissue coverage, and optimal timing of surgery. Reconstructive material can be autologous (i.e., vascularized versus non-vascularized bone) or alloplastic (i.e., titanium, polymethylmethacrylate, polyetheretherketone [PEEK], etc.) [2]. While no evidence currently suggests that one type is superior to the other in terms of symptomatic improvement, autologous bone remains the gold standard for cranioplasty due to its ability to resist infection [4]. Furthermore, chimeric vascularized bone and soft tissue flaps can be utilized to treat composite calvarial defects [4]. With more extensive defects, however, the quantity of autologous bone required to provide adequate structural support may not be available. In these cases, reconstruction with customized alloplastic implants and total vascularized soft tissue coverage offers a potential alternative.
Despite evidence that early surgical intervention improves outcomes in patients with the SoT, high failure rates following immediate cranioplasty for hostile calvarial defects have pushed many practitioners to delay reconstruction for 6 to 12 months [6]. During this period, proper wound bed preparation with serial debridement, local wound care, and sensitivity-directed antibiotic therapy is critical to maximizing the odds of successful reconstruction. In this report, we describe delayed reconstruction of a hostile calvarial defect by utilizing an alloplastic PEEK implant and chimeric microvascular soft tissue coverage in a 48-year-old male with SoT, leading to near-complete cognitive, behavioral, and sensorimotor recovery.
CASE
Informed consent was obtained from the patient’s power of attorney for the writing and publication of this case report. A 48-year-old male who sustained significant intracranial hemorrhage from a fall underwent bifrontal craniectomy with calvarial banking in the anterior abdominal wall. Three months after his initial injury, bifrontal cranioplasty with autologous calvarial bone graft was performed. The patient presented with signs of hydrocephalus 2 months after the procedure. Computed tomography (CT) scan of the head revealed a subdural hygroma, necessitating placement of a ventriculoperitoneal (VP) shunt. Postoperatively, the patient demonstrated residual signs of traumatic brain injury but was able to communicate effectively through speech; interact socially; and participate in activities of daily living, such as ambulation, self-feeding, and basic personal hygiene.
Four years later, the patient presented to our tertiary medical center with fever, chills, and altered mental status. CT scan identified a significant subdural abscess involving his VP shunt as well as osteomyelitis of his native bifrontal cranioplasty. The shunt was removed in conjunction with secondary bifrontal craniectomy, followed by serial debridement and excision of dura overlying the frontal cortices. Intraoperative cultures grew methicillin-resistant staphylococcus aureus, and aggressive, prolonged antibiotic therapy was initiated. Primary closure of the overlying soft tissue was achieved after cranialization of the frontal sinuses to prevent intracranial communication with the nasal cavity.
Three months after removal of the infected cranial bones, the patient experienced a progressive decline in neurocognitive function consistent with the SoT. He was unable to communicate, ambulate, or follow basic commands. Tracheostomy- and gastrostomy-tube placements were performed for airway protection and nutritional supplementation, respectively. The frontal scalp flap appeared sunken, contracted, and adherent to the cranial base, with the resultant calvarial defect measuring approximately 495 cm2 (Fig. 1A). CT scan demonstrated significant compression of the frontal cortex and ventricular system (Fig. 1B). Definitive calvarial reconstruction was performed 5 months after his secondary craniectomy in conjunction with neurosurgery, who performed the dissection of the brain and dura from the overlying structures. Minimal scar tissue was present. A customized PEEK implant (Stryker, Kalamazoo, MI, USA) was used with a chimeric free latissimus dorsi/serratus anterior myocutaneous flap harvested on a single vascular pedicle from the thoracodorsal system (Fig. 2A). End-to-end microvascular anastomosis was performed to the ipsilateral superficial temporal recipient vessels. The serratus muscle was inset along the floor of the anterior cranial base to minimize endocranial dead space (Fig. 2B). The PEEK implant was then secured over the cranial defect with a side burr hole to allow extracranial passage of the serratus vascular pedicle (Fig. 2C). Finally, the latissimus dorsi myocutaneous component was inset into the scalp to provide external coverage of the implant and facilitate postoperative monitoring. Multiple drains were placed subcutaneously beneath the scalp and at the donor site (Fig. 3A).
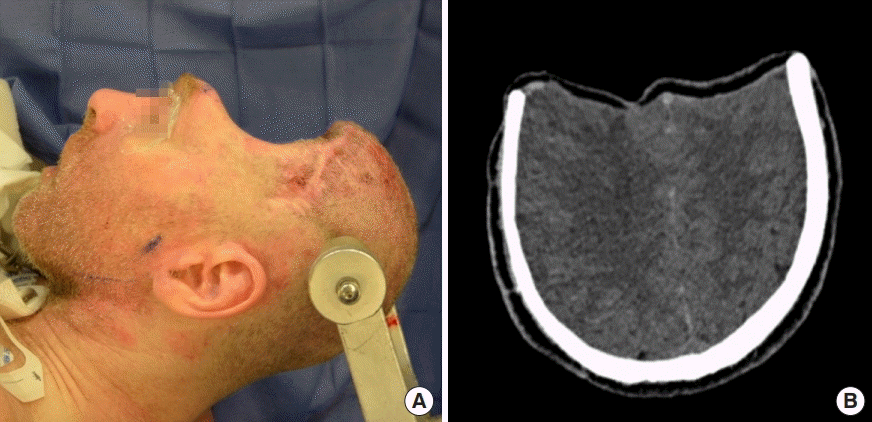
Preoperative cranial defect
(A) Sunken scalp flap measuring 15 cm× 33 cm. (B) Preoperative head computed tomography demonstrating compression of frontal cortices.
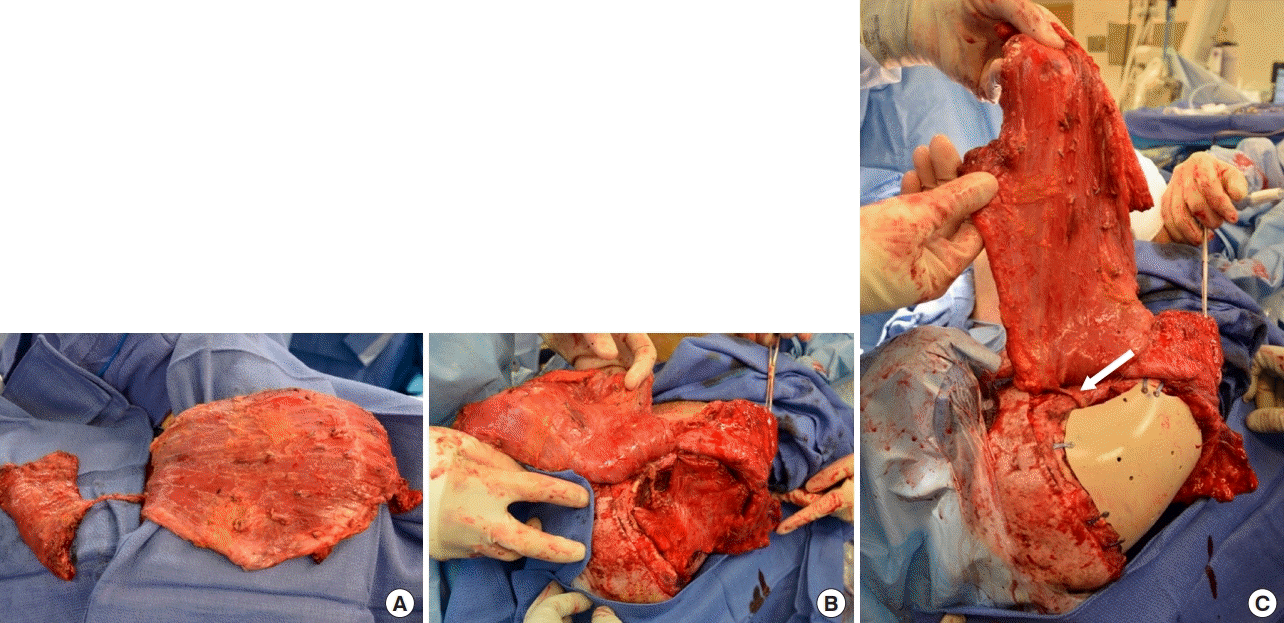
Chimeric flap elevation and inset
(A) Latissimus dorsi and serratus anterior harvest from the same thoracodorsal pedicle. (B) Serratus flap inset along the floor of the anterior cranial base with suture fixation to surrounding periosteum. (C) Polyetheretherketone (PEEK) implant inset with side burr hole to allow extracranial passage of the serratus branch pedicle (white arrow).
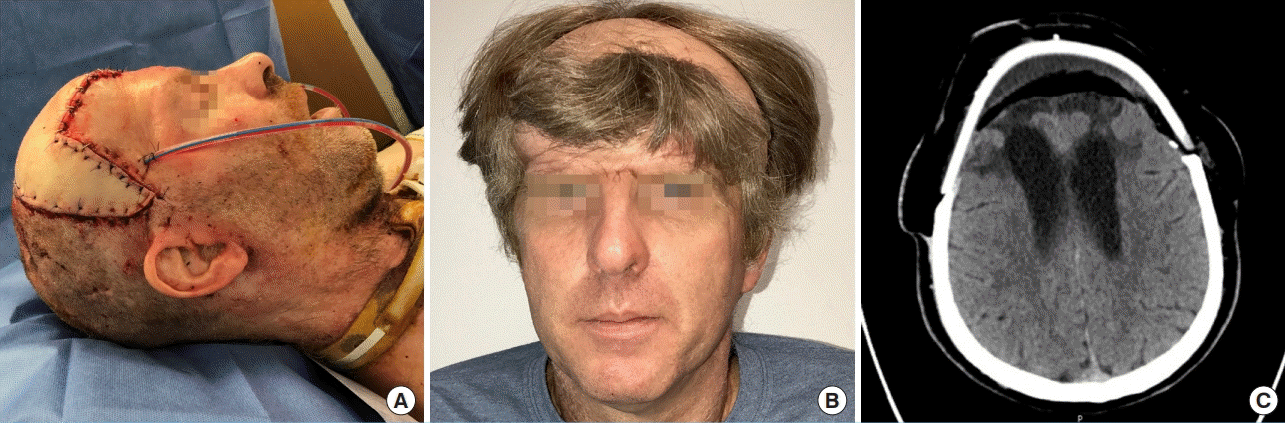
Postoperative result
(A) Immediate postoperative view of alloplastic cranioplasty with vascularized soft tissue coverage, which included a latissimus flap skin paddle for postoperative monitoring. (B) Results at 1 year demonstrating restored frontal contour and a well-healed, viable flap. (C) Head computed tomography at 1 year demonstrating cerebroventricular re-expansion with expected postoperative flap atrophy.
During the first postoperative week, the patient demonstrated marked improvement in his level of alertness, orientation, speech, motor skills, and social interaction. He was able recognize family members and follow simple commands. At the time of discharge (i.e., 4 weeks after surgery), he was able to tolerate a regular diet and ambulate with assistance. Between 6 months to 1 year postoperatively, his incisions healed completely, and CT scan showed cerebral re-expansion without recurrence of subdural abscess (Fig. 3B and C). He demonstrated significant progress with regard to speech, independent ambulation, and ability to engage in activities of daily living. Despite residual difficulty with word recall and short-term memory at 1.5-year follow up, the patient was very satisfied with his functional status and physical appearance.
DISCUSSION
The SoT is a progressive neurologic deterioration that affects 1.2% to 24% of patients with large craniectomy defects resulting from various indications. Loss of intracranial domain generates negative pressure gradients across the brain, leading to cortical compression and alterations in cerebral blood and CSF flow [3]. These effects manifest clinically as a deterioration of sensorimotor, behavioral, and cognitive function [3]. Average time to symptom onset is 5 months post-craniectomy but can span from 3 days to 7 years after cranial bone removal [1,2]. Due to the wide range in timing of presentation and incidence, this syndrome is most likely underdiagnosed and underreported [2]. Prompt evaluation and treatment are critical, as cranial vault reconstruction can resolve the deficits observed in these patients by facilitating cerebral re-expansion and hydrodynamic normalization [2]. The present case demonstrates that delayed, subtotal calvarial reconstruction using combined alloplastic implant and vascularized soft tissue coverage can achieve rapid reversal of neurologic symptoms with restoration of baseline functional status.
The primary goals of cranial vault reconstruction are immediate cerebral protection, re-establishment of intracranial domain, healthy soft tissue coverage, and adequate cosmesis. Current practice guidelines recommend performing cranioplasty 3 months after craniectomy to minimize cerebral edema [2]. Several studies have shown that shorter delay to definitive reconstruction (i.e., less than 85 days) can improve functional outcomes [5,7]. However, for large, hostile defects complicated by infection, irradiation, CSF leak, and/or prior surgery, early cranioplasty may be more prone to failure [4]. In these high-risk patients, careful operative planning with regard to reconstructive material and surgical timing are the keys to success.
Current options for reconstructive material include autologous bone and alloplastic implants. Autologous bone grafts are resistant to infection and cost-effective, but are limited with regard to availability, donor-site morbidity, and contouring difficulty [5,8]. Non-vascularized bone grafts also undergo significant resorption over time [8]. In contrast, alloplastic implants are readily available, mechanically durable, and easy to contour but suffer from susceptibility to infection and extrusion [6]. When the defect involves bone and skin, both types of material can be combined with vascularized soft tissue flaps to optimize scalp perfusion and prevent infection. However, in the setting of a hostile, composite cranial defect, the ideal choice is vascularized autologous bone grafting with microvascular soft tissue transfer.
In the present case, the patient’s wound presented specific challenges due to defect size, history of infection, and presence of endocranial dead space. Timing of the procedure was delayed 5 months after secondary craniectomy to allow for adequate wound bed preparation and antibiotic therapy. The extent of the patient’s calvarial defect (495 cm2) precluded the use of autologous bone, which has been shown to have a 60% failure rate in defects larger than 75 cm2 [9]. Furthermore, a 2013 study of 180 cranioplasty patients found no significant difference in postoperative infection rate between alloplastic and autologous materials [10]. In order to provide sufficient skeletal support and restore frontal contour, we elected to use a customized PEEK implant. Virtually modeled using computer-aided design/computer-aided manufacturing technology to fit the patient’s anatomy, the implant is then milled from a large sheet of polymer via subtractive manufacturing [11]. Compared to other alloplastic candidates such as titanium and polymethylmethacrylate, PEEK has an equivalent infection rate of 7.6% to 13% [12,13]. Like titanium, PEEK is strong, thermoplastic, and resistant to aggressive sterilization; however, PEEK also possesses a bone-like elasticity, allowing it to absorb energy on impact and provide superior intracranial protection [12].
Despite restoration of cranial support with the PEEK implant, prolonged cerebral compression had left the patient with a large endocranial dead space prone to seroma and abscess formation. Kumar et al. [14] found high rates of alloplastic implant failure in patients with endocranial dead spaces and/or poor quality soft tissue coverage. For these reasons, total vascularized soft tissue coverage of the implant may improve success rates following alloplastic remodeling by minimizing dead space and resisting infection. The chimeric latissimus dorsi/serratus anterior myocutaneous free flap, as described in the present case, offers one potential alternative to achieve these goals. The extracranial latissimus flap protects the PEEK device, while the thinner-profile, intracranial serratus flap provides a vascularized surface lining, prevents fluid accumulation, and accommodates cerebral re-expansion.
Favorable outcomes cited within the literature support the neurologic benefits of cranioplasty in the treatment of SoT; however, the exact mechanism by which bony architectural support induces brain re-expansion remains unclear. In a systematic review, Ashayeri et al. [1] reported that noticeable symptom improvement occurred at an average of 3.8 days post-cranioplasty, with 55% of patients achieving functional independence in activities of daily living and 34.6% experiencing complete reversal of neurologic deficits. In a 25-patient study, Honeybul et al. [15] found objective improvement in postoperative cognitive and functional ability scores in 16% of patients. Similar to existing data, our patient demonstrated significant improvement with regard to alertness, orientation, speech, motor skills, and social interaction within the first postoperative week. By the sixth postoperative month, he had returned to his baseline, post-injury neurologic status, regaining the ability to ambulate, feed himself, speak, and engage independently in activities of daily living. CT scan at 1 year demonstrated ventricular re-expansion, consistent with prior reports, along with cerebral-serratus opposition and no evidence of infection recurrence [3,8].
The SoT is a neurological phenomenon characterized by sensorimotor, cognitive, and behavioral deficits that occur following the violation of intracranial pressure in patients with large craniectomy defects. The most effective treatment strategy for improving or reversing these neurologic sequelae is early cranioplasty, which re-establishes intracranial domain and normalizes cerebral hydrodynamics. In the setting of a hostile wound bed, autologous reconstruction is the gold standard to prevent infection. However, in the case of extensive, high-risk, and/or composite cranial defects, early cranioplasty with autologous bone may not be feasible. In the present report, we demonstrated the efficacy of delayed cranial vault reconstruction using an alloplastic implant, in combination with total vascularized soft tissue coverage, with excellent functional recovery and no evidence of recurrent infection on long-term follow-up. Based on our findings, we advocate this approach as a reasonable alternative for the treatment of hostile, subtotal calvarial defects in patients with SoT.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of his images.
Author contribution
Writing and editing: Wang JS. Writing: Ter Louw RP. Editing: DeFazio MV. Supervision. McGrail KM, Evans KK. Approval of final manuscript: all authors.
