Optimal Initial Dose of Chloral Hydrate in Management of Pediatric Facial Laceration
Article information
Abstract
Background
Chloral hydrate (CH) is the primary agent most commonly used for pediatric sedation prior to diagnostic, therapeutic procedures. In the management of pediatric facial laceration, the initial dose of CH has to balance the need for adequate sedation against the need to minimize sedative complications.
Methods
A retrospective review of medical records of 834 children who visited our emergency room for facial lacerations from August 2010 to September 2012 was conducted. They were divided into six groups on the basis of the initial dose of CH administered. Further, each group was compared with the standard group (70 to ≤80 mg/kg) with respect to sedation success, augmentation dose, failed sedation, time to procedure, and time of stay.
Results
With respect to the complication rate, only group 1 (range, 40 to ≤50 mg/kg) showed a significantly lower complication rate. In the case of all the other variables considered, there were no significant differences among any of the groups.
Conclusions
An initial CH dose of 48±2 mg/kg does not negatively affect the success rate of sedation or the need for additional sedative during the primary closure of facial lacerations in pediatric patients. Further, lower doses reduce the incidences of adverse effects and do not delay procedure readiness. Therefore, 48±2 mg/kg of CH can be considered the optimal initial dose for pediatric sedation.
INTRODUCTION
Chloral hydrate (CH) is one of the most commonly used sedatives, and usually, CH is the primary agent used for pediatric sedation prior to diagnostic procedures, such as computed tomography, magnetic resonance imaging (MRI), echocardiography, and electroencephalography (EEG) [1]. In our plastic surgery department, CH is used for sedating children that need primary repair of facial lacerations in an emergency room. Unlike diagnostic procedures, primary repair is preceded by a painful pre-sedational injection of local anesthetics that may distress children before the induction of sedation. Furthermore, children may experience various mechanical stimuli during procedures. Although this higher level of stimulus might increase dose requirements, little information is available in the existing literature on the topic [2].
CH is a safe sedative with a low complication risk. Nevertheless, its potential adverse effects range from gastric distress, nausea, and vomiting that are mild and self-limited, to effects such as ataxia, lethargy, deep coma, respiratory depression, hypotension, and cardiac arrhythmias that are potentially life threatening [3]. The average half-life of trichloroethanol, the first metabolite of CH and responsible for CH's pharmacological activity, is 8 hours (range, 7-9.5 hours) [4], and this long half-life may cause late re-sedation and other adverse effects after discharge. In the practical environment, despite the possibilities of re-sedation and other late adverse events, careful observation over the half-life of CH is unrealistic, particularly in the emergency room setting. Therefore, the initial dose should be as small as possible, as long as it is effective.
Thus, in view of the conflicting requirements of successful sedation for primary repair and the minimization of possible complications, guidance is required with respect to the optimal CH dose. In the existing literature and in pediatric textbooks, 50 to 100 mg/kg of CH is generally recommended, and 75 mg/kg is usually viewed as a standard dose in a number of publications [2,5-9]. Accordingly, we aimed to identify an optimal initial CH dose for pediatric sedation for primary repair by comparing the findings of patients who were given different CH doses.
METHODS
This study was conducted by retrospectively reviewing the medical records of 834 children who visited our emergency room for facial lacerations from August 2010 to September 2012. The sequence of procedures performed on the children was as follows: wound irrigation, injection of local anesthetics (lidocaine), administration of sedative, and wound suture in that order. These children were divided into six groups on the basis of the recorded initial dose range of CH administered in ascending order, as follows: group 1, 40 to ≤50 mg/kg; group 2, 50 to ≤60 mg/kg; group 3, 60 to ≤70 mg/kg; group 4, 70 to ≤80 mg/kg; group 5, 80 to ≤90 mg/kg; and group 6, 90 to ≤100 mg/kg. Group 4, which included the standard dose (75 mg/kg), was regarded as the standard group and was compared with the other groups with respect to sedation success, augmentation dose, failed sedation, time to procedure, and time of stay.
Categorical data, such as the incidence of adverse events and sedation failure, were analyzed using Fisher's exact and chi-squared tests as appropriate. Continuous data, such as age, weight, medication dosage, time to procedure, and time of stay, were analyzed using one-way analysis of variance with Dunnett's multiple comparisons test. P-values of <0.05 were considered significant.
Definition of terms used in this study
The following definitions were considered: 1) Sedation failure: sedation deemed inadequate after the administration of initial or of an augmentation dose of CH, finally resulting in the inability to perform primary repair without physical restraint. 2) Sedation success: sedation conducted uneventfully without sedation failure during the procedure. Transient awakening or irritability followed by successful re-sedation was also viewed as sedation success. 3) Augmentation dose: the additional administration of CH due to inadequate sedation after initial administration. 4) Time to procedure: the time in minutes from the administration of CH to the documented time of a procedure. 5) Time of stay: the time in minutes from the administration of CH to the documented time of discharge.
RESULTS
The demographics of the 834 children are summarized in Table 1. Five hundred and fifty-five (66%) of these children were male, and all received CH sedation for the primary repair of facial lacerations in an emergency room. The mean subject age was 30 months (range, 4-106 months), and the mean weight was 13.3 kg (range, 5-28 kg). With the exception of fever (>37.5℃, 13 children), there were no significant comorbidities. The mean CH doses in the six groups (in an ascending order) were 48, 55, 66, 76, 85, and 97 mg/kg, respectively.
The results of statistical analyses of sedation success and augmentation dose are shown in Table 2. No significant differences were found. However, in groups 5 and 6, fewer children tended to need augmentation without statistical significance. On the other hand, no increase in the need for augmentation was observed in groups 1 to 3 (Fig. 1).
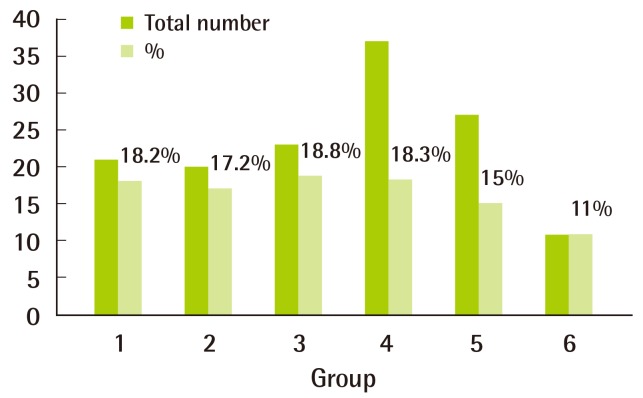
Proportion of children requiring augmentation dose
In groups 5 and 6, fewer children tended to need augmentation, but the difference was not statistically significant.
A summary of the statistical analysis of the complication types is provided in Table 3. In the decreasing order of frequency, the complications encountered were vomiting, irritability, and desaturation. In group 1, the overall frequency of complications was significantly lower than that in group 4 (the standard). However, no significant differences were observed among the other groups.
The results of our analyses of time variables are shown in Table 4. No significant intergroup difference was found between any two groups.
DISCUSSION
Our study shows that an initial CH dose of 48±2 mg/kg does not negatively affect the success rate of sedation or the need for additional sedative during the primary closure of facial lacerations in pediatric patients and that lower doses reduce the incidences of adverse effects and do not delay procedure readiness.
High efficacy, low complication rates, and the ease of administration support the widespread use of CH. In previous studies, various doses of CH between 50 and 100 mg/kg (up to a maximum of 2 g or 100 mg/kg, whichever is the lowest) have been used, and efficacies of <92% for the initial doses of 70, 75, and 100 mg/kg have been reported [2,6,7,10,11]. In the present study groups, efficacies ranged from 97% to 100%, which is consistent with previous reports (Table 2). Sedation failure rates were reported to decrease from 15.5% to 5.5% in the case of CH augmentation [12]. Of the children included in the present study, 11% to 18.8% were given CH augmentation because of inadequate sedation by the initial CH dose; this decreased the sedation failure rate to 0% to 3% (Table 2).
CH sedation is associated with two types of adverse effects. First, adverse effects caused by irritation of mucous membranes, such as gastric distress, nausea, and vomiting, and second, adverse effects caused by CH overdose, which include ataxia, lethargy, deep coma, respiratory depression, hypotension, and cardiac arrhythmia [3]. In previously published studies, the rates of adverse effects were as follows: vomiting, 2% to 30%; irritability, 1% to 6%; and desaturation, 0% to 4% [13-17]. Similar frequencies were found in the present study, and most of the adverse effects were of the first type (Table 3). However, the lower dose group had a lower rate of adverse effects of the first type.
Overdose can be prevented by precisely measuring the CH syrup and the weight of the child. In the case of a child who has vomited, it is difficult to decide on the level of augmentation. In particular, when the initial dose administered is a dose that is close to the upper limit dose, augmentation is associated with a considerable risk of adverse effects. Thus, a lower initial dose of CH provides greater latitude when considering augmentation in a child who has vomited.
The possibility of re-sedation due to the long half-life of the active metabolite of CH has been well discussed in the existing literature. Cote et al. [18] and Malviya et al. [19] noted that only 48% of the children in their study returned to baseline activity and behavior within 8 hours and that 89% returned to baseline within 24 hours. Notably, 5% of all children did not return to baseline activity levels until the second day after the procedure. The adverse effects reported after discharge include motor imbalance (31%), gastrointestinal effects (23%), agitation (19%), and restlessness (14%) [18,19]. Accordingly, prolonged recovery and the high incidence of post-discharge adverse effects support the administration of as low a dose of CH as possible.
In an effort to reduce the possible adverse effects but retain efficacy, Bracken et al. [8] administered 20% less CH than their standard dose (75 mg/kg) and found that this reduction had no negative effect on the outcome or the time to sedation. In the present study, the sedation success rate, time to sedation, and time of stay were almost identical for all the study groups, despite the significantly lower complication rate of group 1 (48±2 mg/kg).
The need for pre-sedational fasting to reduce the risk of aspiration has been losing ground. Keidan et al. [12] showed that fasting was associated with an increased failure rate of initial sedation and found that fasting increased the total dose of CH required and this in turn increased the sedation time. Further, unlike in elective diagnostic procedures such as MRI and EEG, an injured child visits a medical institution due to an accident and are likely not to have fasted in advance. Thus, in the present study, we did not require the children to fast prior to CH sedation [12,20].
Notes
No potential conflict of interest relevant to this article was reported.



