Subeschar culture using a punch instrument in unstageable wounds
Article information
Abstract
Background
A patient’s overall condition sometimes does not allow for the complete removal of a dead eschar or injured slough in cases involving a pressure-injury skin lesion. This frequently occurs in clinical practice, particularly in bedridden and older patients receiving home care or intensive care. Even after debridement, it is also difficult to manage open exudative wounds in these patients. Nevertheless, when a mature or immature eschar is treated without proper debridement, liquefaction necrosis underneath the eschar or slough tends to reveal a large, open wound with infectious exudates. We hypothesized that if the presence of any bacteria under the eschar can be evaluated and the progression of the presumed infection of the subeschar can be halted or delayed without creating an open wound, the final wound can be small, shallow, and uninfected.
Methods
Using a punch instrument, we performed 34 viable subeschar tissue cultures with a secure junction between the eschar and the normal skin.
Results
The bacterial study had 29 positive results. Based on these results and the patient’s status, appropriate antibiotics could be selected and administered. The use of suitable antibiotics led to relatively shallow and small exposed wounds.
Conclusions
This procedure could be used to detect potentially pathogenic bacteria hidden under black or yellow eschars. Since subeschar infections are often accompanied by multidrug-resistant bacteria, the early detection of hidden infections and the use of appropriate antibiotics are expected to be helpful to patients.
INTRODUCTION
There is clinical consensus that debridement is necessary for wound bed preparation. Even though very few direct human studies of pressure ulcer debridement are available to support these recommendations, debridement in cases with adequate wound bed vascularity is considered critical in wound bed preparation, as it not only addresses obstacles to the healing of chronic wounds, but also may provide stimulatory effects [1].
The treatment of wounds that include dead tissue generally involves removal of the dead tissue with subsequent exposure of any viable tissue with pinpoint bleeding. Healing by primary, secondary, or tertiary intention is then selected according to the size and condition of the exposed defect or wound. Skin grafts, local flaps, and free flaps are the main options considered when the adjacent tissue is difficult to suture.
In contrast, unstageable wounds involve full-thickness tissue loss with the ulcer base covered by slough (tan, yellow, green, brown, or gray) and/or eschar (brown, tan, or black) in the wound bed. It is not possible to determine the true depth, and therefore the stage, of the wound until sufficient slough or eschar has been removed to expose the base of the wound. According to the guidelines published by the European Pressure Ulcer Advisory Panel (EPUAP) and the National Pressure Ulcer Advisory Panel (NPUAP), stable—dry, adherent, and intact without erythema or fluctuance—eschars on the heel should not be removed [2]. In addition, a patient’s general condition sometimes does not allow for the removal of the entire dead eschar or injured slough in the case of a pressure-injury skin lesion.
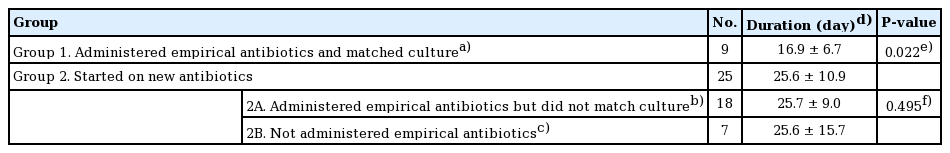
When debridement or escharectomy is performed in these patients, it is also difficult to manage the resulting open exudative wounds. In a patient with a mature (totally necrotized) or immature (necrotizing) eschar, if only conventional treatment is performed, liquefaction necrosis underneath the eschar reveals a large, open, deep wound with infectious exudates. This frequently occurs in clinical practice, particularly in bedridden and older patients receiving home care or intensive care (Fig. 1).
Surface color change of a pressure ulcer
For patients on a ventilator who are unable to move by themselves, it is difficult to avoid skin damage despite frequent changes in position. When accompanied by excessive moisture, such as sweat or urine, the skin peels off, and a yellowish eschar can be seen (upper row). In the case of dry skin, necrosis progresses, and a dark-colored eschar is visible (lower row).
Therefore, if the presence of pathogens under the eschar can be assessed and the progression of the potential subeschar infection can be halted or delayed without escharectomy, we expect the final wound to be small and shallow. We attempted to perform subeschar viable tissue cultures with a secure junction between the eschar and the normal, periwound skin.
METHODS
We reviewed 34 patients referred to us for unstageable lesions on the sacrococcygeal area due to pressure injuries in the intensive care unit between 2011 and 2018. Although the staff of the intensive care unit attempted to prevent pressure ulcers in all patients, the development of these ulcers usually cannot be prevented entirely. Most of the patients had been referred to the plastic surgery department for wound care when discoloration of the pressure-injured skin lesion was noticed. Patients’ age, sex, underlying diseases, admission period, duration of antibiotic usage, and bacteriological study results were examined, and previously prescribed antibiotics were noted. The patients agreed to this study and provided written informed consent for the publication and the use of their images. This study was approved by the bioethics committee of our institution under institutional review board protocol (IRB No. CR-19-123).
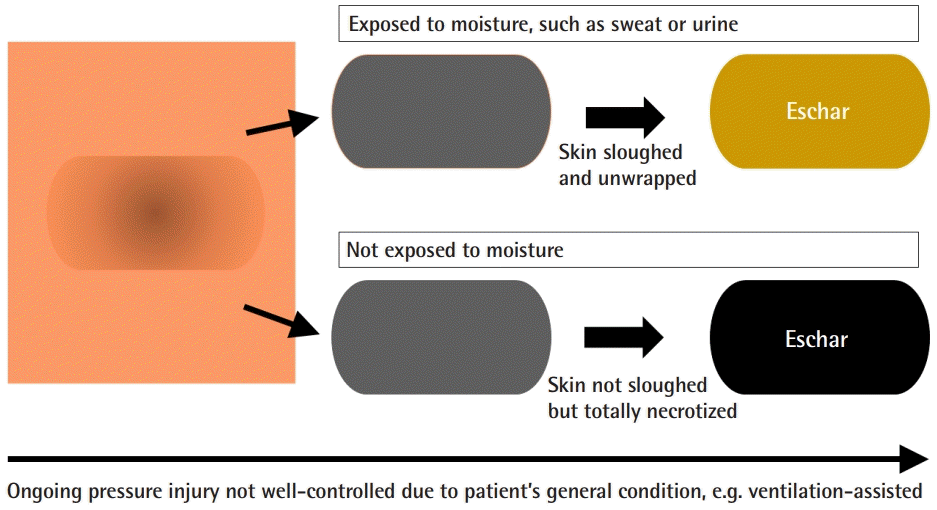
Sterile gloves, punch biopsy instruments (4-mm biopsy punch; sfm medical devices GMbH, Wächtersbach, Germany), toothed Adson forceps, Metzenbaum scissors, sterile fenestrated drapes, and labeled sterile carrier tubes were prepared for the biopsies. The toothed Adson forceps were required to elevate the specimens, while the Metzenbaum scissors were needed to cut the specimens, first from the base and then from the eschar.
Punch subeschar tissue culture (PSTC) without escharectomy was performed as illustrated in Fig. 2. First, the areas to be biopsied were scanned. Commonly selected biopsy sites were those adjacent to the darkest site of discoloration in the eschar or the slough. Sterile, powder-free gloves were worn after proper hand hygiene measures were taken. The PSTC procedure was repeated every week until the result was negative. At that point, we recommended that the physician stop the antibiotics prescribed for the wound. The bacterial study and sensitivity test results took 5 to 7 days. A proper antibiotic could then be selected based on the sensitivity test and on the patients’ underlying diseases, such as kidney or liver diseases.
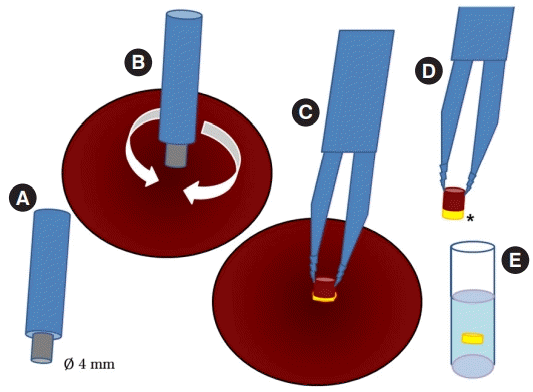
Method of collecting uncontaminated subeschar tissue
(A) Sterilized punch biopsy instruments (diameter, 4 mm) are prepared. Anesthesia is not required due to the potential for liquefaction necrosis to have incapacitated the sensory nerves. (B) The periwound skin and eschar are cleansed thoroughly with povidone iodine solution. The most deeply darkened area of the eschar, which is the area of deepest injury, is chosen for biopsy using a sterilized punch biopsy instrument. The endpoint of the punch depth is when the 1- to 2-mm-thick discolored soft tissue is exposed and seen under the eschar. (C, D) The cylindrical specimen is lifted from the hole made by the punch instrument with sterilized toothed forceps. The base of the tissue column is cut with the distal part of sterilized Metzenbaum scissors. The soft subeschar yellowish tissue sample (*) is separated from the eschar with new sterilized Metzenbaum scissors or with the inner proximal part of the previous sterilized Metzenbaum scissors to avoid the effect of the remaining antiseptic chemicals. (E) Separated subeschar tissue is moved into a culture tube with one or two sterile cotton stick(s). The dimension of the subeschar tissue is usually 1 to 2 mm. The wounds may not need to be closed with any sutures. Commercial antibiotic ointment should be applied twice a day, and the entirety of the eschar and periwound area is covered with sterile gauze to prevent secondary infection.
Twenty-seven of the patients were treated with an empirical antibiotic (second-generation cephalosporin) prior to the procedure, while the remaining seven patients were not. Based on the PSTC results, the patients on empirical antibiotics were either kept on the empirical drug they had previously been taking or were administered a new antibiotic. We compared the duration of antibiotic use between these groups. In addition, the patients who were previously being treated with empirical antibiotics and were switched to a new antibiotic based on the PSTC results were compared with those who were not previously treated with empirical antibiotics. Statistical analysis was performed using IBM SPSS statistics version 19.0 (IBM Corp., Armonk, NY, USA). A comparison with regard to the use of prophylactic antibiotics was carried out using the Mann-Whitney U test. P-values of less than 0.05 were considered to indicate statistical significance.
RESULTS
All patients were in poor overall condition, were unable to undergo surgery, and could not change positions by themselves. Among the 34 patients, 25 were in the intensive care unit with airway intubation and multi-organ failure, four with cardiovascular and respiratory problems, three with terminal cancer, and two with neurological problems (Table 1).
Three referred patients had fever, 10 had a local sensation of heat, and 11 had erythematous changes around the eschar, but none initially had any exudate from the wound. The first PSTCs showed results of Staphylococcus aureus (n = 13), Escherichia coli (n = 7), Pseudomonas aeruginosa (n = 5), Acinetobacter baumannii (n = 4), and a lack of bacterial growth (n = 5).
Treatment with suitable antibiotics was continued until cultures were no longer positive following PSTC reports. Among all 34 patients, the mean duration of suitable antibiotic treatment was 23.3 days. The mean duration of antibiotic treatment in the patients treated with empirical antibiotics who remained on the empirical drug (n = 9) was 16.9 ± 6.7 days, while that of the remaining patients (n = 25), who were switched to new antibiotics according to the PSTC results, was 25.6 ± 10.9 days. In the second group, the mean duration of the 18 patients who were previously treated with empirical antibiotics was 25.7 ± 9.0 days, and that of the seven patients who had no prophylactic antibiotic treatment was 25.6 ± 15.7 days.
The difference in the duration of antibiotic use according to the replacement or continuation of antibiotics due to the results of the culture was statistically significant (Table 2). Statistical analysis was performed using the Mann-Whitney U test, since the Shapiro-Wilk test showed that the sample did not follow a normal distribution.
The size of each wound was no larger than that of the initial lesion after wound contraction, and the depth was no greater than stage III or IV (Fig. 3).
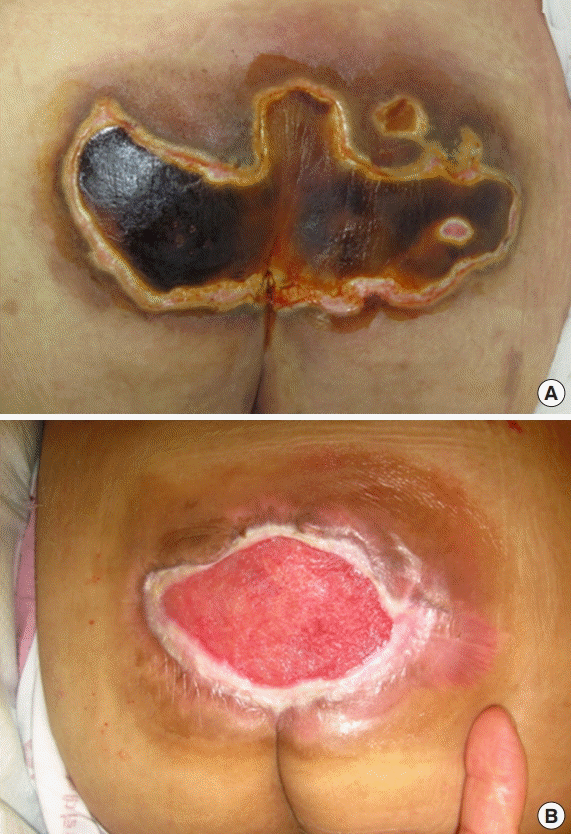
A case of an unstageable large wound
The patient with this large eschar on the sacrococcygeal area was bedridden and on a ventilator in the intensive care unit. At first, some erythematous changes and a localized sensation of heat were noted around the eschar. (A) The small open wound on the right side in the middle of the eschar lesion was the site of the punch subeschar tissue culture. The infection was controlled with proper antibiotics chosen based on the sensitivity result of the bacteriological study. (B) Due to proper antibiotic therapy, this pressure-injury lesion wound stabilized at stage II with marginal contraction.
DISCUSSION
Older adults in long-term care settings represent a population that is particularly vulnerable to pressure ulcers due to factors such as poor nutrition, loss of cognitive function, immobility, and incontinence. Even in health systems, despite the improvements associated with the Braden pressure ulcer risk assessment scale, pressure damage still occurs in unconscious patients, patients with brain or spinal nerve damage, patients in critical condition, and patients with general weakness [3]. Clinical studies have shown a 100-fold increase compared to control groups in the number of bacteria in an inoculated wound that is subjected to pressure [4,5]. The wound bed may be covered with necrotic tissue (tissue that has become nonviable due to reduced blood supply), slough (dead tissue, usually cream or yellow in color), or eschar (dry, black, hard necrotic tissue). The presence of such tissue impedes healing. Necrotic tissue and slough may be quantified as excessive (+++), moderate (++), minimal (+), or absent (−). Since necrotic tissue can also carry pathogenic organisms, the removal of such tissue helps to prevent wound infection. To facilitate healing, the necrotic tissue and slough should be debrided with a scalpel so that the wound bed can be accurately assessed. An eschar may adhere to the wound bed, making debridement with a scalpel difficult. Further debridement for the purpose of wound management may be required using other techniques [6].
In some specific conditions, specifically in inoperable patients or when a large open wound is present with exudates after necrotic tissue removal in patients with urinary or bowel incontinence, proper wound management is difficult. When a hidden infection is suspected in a wound covered with an eschar, the first choice of treatment is to remove the necrotic tissue through a surgical technique called escharectomy and to perform a bacterial culture.
In an open wound, the wound healing mechanism produces a clot containing platelets and fiber. This protects the wound from contamination and pathogens and promotes epithelization under the wound [7]. However, pressure ulcers undergo a different healing process from normal open wounds, and the mechanism of the former is still unclear. In pressure sore wounds from which a necrotic eschar is removed, an open wound is typically observed along with liquefactive necrosis. This is a type of necrosis in which tissues are transformed into viscous liquid [8]. In such cases, local bacterial or fungal infections are often present. Cells that undergo liquefactive necrosis are completely decomposed by hydrolytic enzymes, leaving behind a residue of pus and necrotic tissue. The dead white blood cells manifest as creamy, yellow pus. After the white blood cells are removed, the liquid-filled space remains as an abscess. Skin barriers, eschar, and the loss of necrotic tissue left in the wound provide an environment ripe for bacterial growth and invasive infection.
A report was published on the detection of bacteria via needle biopsy at subeschar sites suspected of infection, but the modality proved ineffective due to difficulty of discerning the needle tip depth as well as false-negative results [9]. Additionally, swab culturing is not recommended, as it can only indicate surface contamination. Instead, an examination can be conducted through debridement of necrotic tissue or through swabs of the gap between necrotic lesions and normal scar tissue. Debridement is relatively invasive, and with appropriate swab use, the process can continue until the necrosis has progressed substantially [10]. Both approaches make the wound of the pressure sore deeper than the necrotic tissue, potentially requiring an even larger operation in the future.
Deep second-degree or third-degree burn wounds covered with eschars are usually treated by removing dead tissue through escharectomy, diminishing the negative effects of the peroxidase-rich subeschar environment. Nevertheless, for pressure ulcers that are covered by extensive eschars in patients in a poor general condition, escharectomy is not recommended by the EPUAP or the NPUAP. According to some previous studies, subeschar infections that appear under these wounds with extensive eschar can proceed rapidly without proper antibiotic treatment [5,7-11]. The bacteriological findings of the subeschar tissue indicate that suitable antibiotics can be chosen based on the pathogen colonies present and the results of the sensitivity test. Many studies have shown that when a patient is in poor general condition, an infection in the subeschar tissue may proceed to sepsis, threatening the patient’s life. Therefore, suitable antibiotics should be used for multidrug-resistant bacteria, such as Acinetobacter and Pseudomonas, when infection is suspected in the subeschar tissue. However, in unclassified pressure sore wounds accompanied by eschar and black necrotic surfaces, antibiotics tend to be underused, worsening the condition of the eschar further [11-16]. To prevent deterioration, infection, discomfort, pain, exudate, and odor, it is necessary to recognize what is happening underneath the stable or immature eschar. Here, we attempted to harvest viable subeschar tissue cultures while maintaining the integrity of the area between the eschar and the normal skin.
We used a sterile medical punch instrument to harvest subeschar tissue by punching through the hard tissue to reach the soft, manhole-cover-like eschar (Fig. 2). This PSTC procedure did not require analgesics or anesthetics. As such, while it may appear invasive, this punch tissue culture could be performed in home care or hospice settings. It requires a sterile environment, achieved via cleansing with povidone iodine. A punch skin biopsy is a well-known technique to obtain diagnostic full-thickness skin specimens for investigating neoplasms, pigmented lesions, inflammatory lesions, and chronic skin disorders [17].
The patients were divided into two groups based on their antibiotic treatment according to the sensitivity test conducted after PSTC. The first group contained patients who were treated with the same antibiotic that they were previously taking, while the second group included patients who were administered new antibiotics. The difference between the two groups was statistically significant, and the total number of days of antibiotic use was found to be lower in the group that continued the same antibiotic. This indicates that empirical antibiotics could be sensitive to potential subeschar pathogens. The group switched to a new antibiotic was further divided into a subgroup of patients who previously used empirical antibiotics and a subgroup of patients who did not. There was no statistically significant difference in the number of days of antibiotic use between these two subgroups. This clearly indicates that using empirical antibiotics to which the bacteria causing the subeschar infection were unsensitive did not have a significant effect on the number of days of antibiotic use or relief of clinical manifestations (Table 2).
Therefore, when subeschar infection is suspected in unstageable wounds with extensive eschar, and the patient’s condition prevents procedures such as invasive escharectomy, even empirical antibiotics may be helpful in the treatment of the wound (26.5% of patients). However, we recommend conducting a bacterial study of the viable tissue below the slough or eschar of unclassified or unstageable pressure injuries using the sterile PSTC procedure. This PSTC method showed positive results in 85.29% of cases (29/34). Hidden, potentially pathogenic bacteria could be found under black or yellow eschars; therefore, we can improve wound prognosis by creating an aseptic subeschar environment through early detection of the causative bacteria and rapidly switching to selective antibiotics to which the bacteria are susceptible (Fig. 4). Early, proper antibiotic usage is needed to prevent progression to sepsis. We believe the method detailed in this study to be useful for the early diagnosis of subeschar infection when a patient cannot undergo surgery. Surgical treatment should be considered if the patient’s condition improves and escharectomy becomes possible.
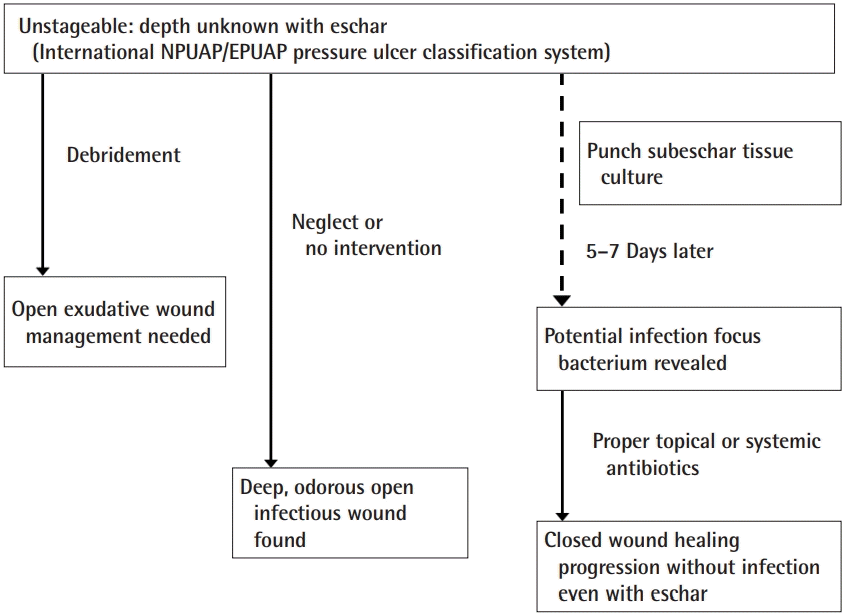
Treatment options for an unstageable pressure ulcer
Under the conventional approach, total escharectomy would a large open wound with exudates, the management of which could be messy. If the wound is neglected or no aggressive intervention is carried out, the injured area can become a large, foul-smelling, infected wound. If a bacteriological study is only then initiated, the proper treatment may be too late. Finally, if punch subeschar tissue culture is performed at the beginning of the progression of this unstageable wound, the proper antibiotics could be chosen 7 days later. NPUAP, National Pressure Ulcer Advisory Panel; EPUAP, European Pressure Ulcer Advisory Panel.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Daegu Catholic University Medical Center (IRB No. CR-19-123) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: YJ Lee. Data curation: YJ Lee, HB Jung. Formal analysis: YJ Lee. Methodology: YJ Lee, HB Jung. Project administration: YJ Lee, HB Jung. Visualization: YJ Lee, HB Jung. Writing - original draft: YJ Lee. Writing - review & editing: YJ Lee.