Thin and superthin perforator flap elevation based on preoperative planning with ultrahigh-frequency ultrasound
Article information
Abstract
The ability to directly harvest thin and superthin perforator flaps without jeopardizing their vascularity depends on knowledge of the microsurgical vascular anatomy of each perforator within the subcutaneous tissue up to the dermis. In this paper, we report our experience with ultrahigh-frequency ultrasound (UHF-US) in the preoperative planning of thin and superthin flaps. Between May 2017 and September 2018, perforators of seven patients were preoperatively evaluated by both ultrasound (using an 18-MHz linear probe) and UHF-US (using 48- and 70-MHz linear probes). Thin flaps (two cases) and superthin flaps (five cases) were elevated for the reconstruction of head and neck oncologic defects and lower limb traumatic defects. The mean flap size was 6.5×15 cm (range, 5×8 to 7.5×23 cm). No complications occurred, and all flaps survived completely. In all cases, we found 100% agreement between the preoperative UHF-US results and the intraoperative findings. The final reconstructive outcomes were considered satisfactory by both the surgeon and the patients. In conclusion, UHF-US was found to be very useful in the preoperative planning of thin and superthin free flaps, as it allows precise anticipation of very superficial microvascular anatomy. UHF-US may represent the next frontier in thin, superthin, and pure skin perforator flap design.
INTRODUCTION
Traditional perforator flaps cannot always fulfill reconstructive needs when the main goal is resurfacing, rather than filling space or creating volume. To overcome this limitation, thin flaps are becoming increasingly popular [1-15].
In traditional preoperative planning, studies of perforator microvascular anatomy are confined to determining the perforator’s point of emergence from the muscular fascia, along with its caliber and velocity, by using both computed tomography angiography and color Doppler ultrasound (US). In cases where thin and superthin flaps are used, no reliable methods have been reported for precise evaluation of the perforator anatomy when the perforator pierces both the Scarpa and the superficial fascia.
The aim of this paper is to report our experience with ultrahigh-frequency US (UHF-US) in the preoperative examination of perforator microvascular anatomy within the superficial fascia. This use of UHF-US aided in preoperative perforator selection and intraoperative direct elevation of thin and superthin flaps.
IDEA
Between May 2017 and September 2018, the perforators of 14 flap donor sites of seven patients were preoperatively evaluated by both US using an 18-MHz linear probe (MyLab 50 X-Vision; Esaote, Genoa, Italy) and UHF-US using 48-MHz and 70-MHz linear probes (Vevo MD; FUJIFILM VisualSonics, Toronto, ON, Canada) in B-mode and color Doppler mode at Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS in Rome. All patients consented to the use of their data for academic and research purposes. Institutional review board approval was obtained for this study (ID#3051).
Using the 18-MHz probe, perforators were first mapped in the selected donor-site area to evaluate the vessel caliber and point of emergence from the muscular fascia. The selected perforators were further studied using 48- and 70-MHz probes to evaluate the depth of the superficial and Scarpa fascia, the thickness of the adipose tissue layers, and the type of vascular arborization of the perforator throughout the adipose tissue up to the dermis, the last of which is not always clearly discernable with an 18-MHz probe. Vascular arborization of the perforator was classified according to Fig. 1.
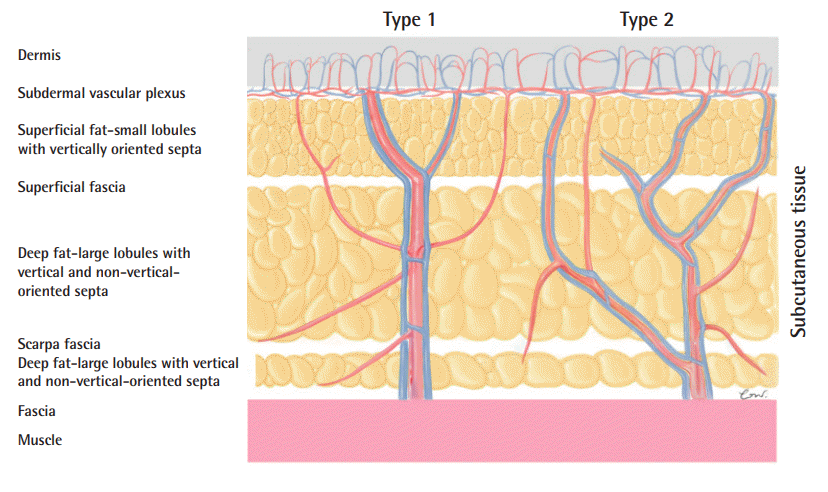
Illustration of perforator types
Schematic illustration of the perforator course within the adipose tissue after piercing the muscular fascia. Type 1 perforators take a direct course from the muscular fascia to the dermis, preserving their caliber until the superficial fascia, where they typically branch. The branches visibly enter the dermis and connect to the dermal plexus vascular network. Type 2 perforators preserve their caliber from the muscular fascia to the Scarpa fascia, where they begin to arborize into collateral vessels within the subcutaneous tissue and finally reach the dermis via smaller branches.
On UHF-US, type 1 perforators take a direct course from the muscular fascia to the dermis, preserving their caliber until they reach the superficial/Camper fascia, where they typically branch. Those branches clearly enter the dermis and connect to the dermal plexus vascular network. In contrast, type 2 perforators preserve their caliber from the muscular fascia to the Scarpa fascia, where they begin to arborize into collateral vessels within the subcutaneous tissue (Supplemental Video 1). Such perforators cannot be easily followed within the subcutaneous tissue with 48- and 70-MHz probes. Finally, unlike type 1 perforators, type 2 perforators do not take a detectable direct course from the muscular fascia to the dermis.
Direct thin free flaps (in two cases, both of which were superficial circumflex iliac artery perforator [SCIP] flaps) and direct superthin free flaps (in five cases: four SCIP flaps and one anterolateral thigh [ALT] flap) were elevated from a single perforator for the reconstruction of head and neck oncologic defects and lower limb traumatic defects (Figs. 2, 3). Intraoperative indocyanine green angiography was used to confirm flap viability before transfer.
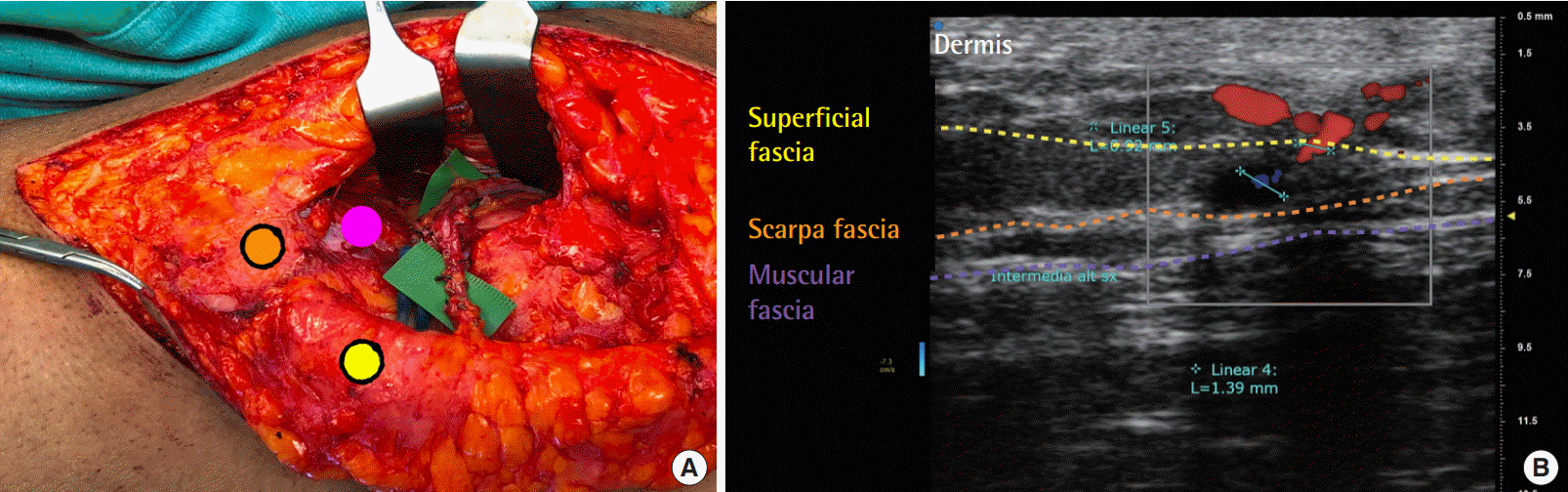
Example of high-fidelity nature of ultrasound findings
Relationships between ultrahigh-frequency ultrasound (UHF-US) results and intraoperative findings. (A) Intraoperative image of a superthin anterolateral thigh perforator flap. Yellow dot indicates superficial fascia, orange dot the Scarpa fascia, and the pink dot the muscular fascia. (B) High-fidelity microvascular planning using UHF-US. The caliber of the perforator was preserved from the muscular fascia to the superficial fascia. The yellow dotted line indicates the superficial fascia, the orange dotted line denotes the Scarpa fascia, and the violet dotted line indicates the muscular fascia.
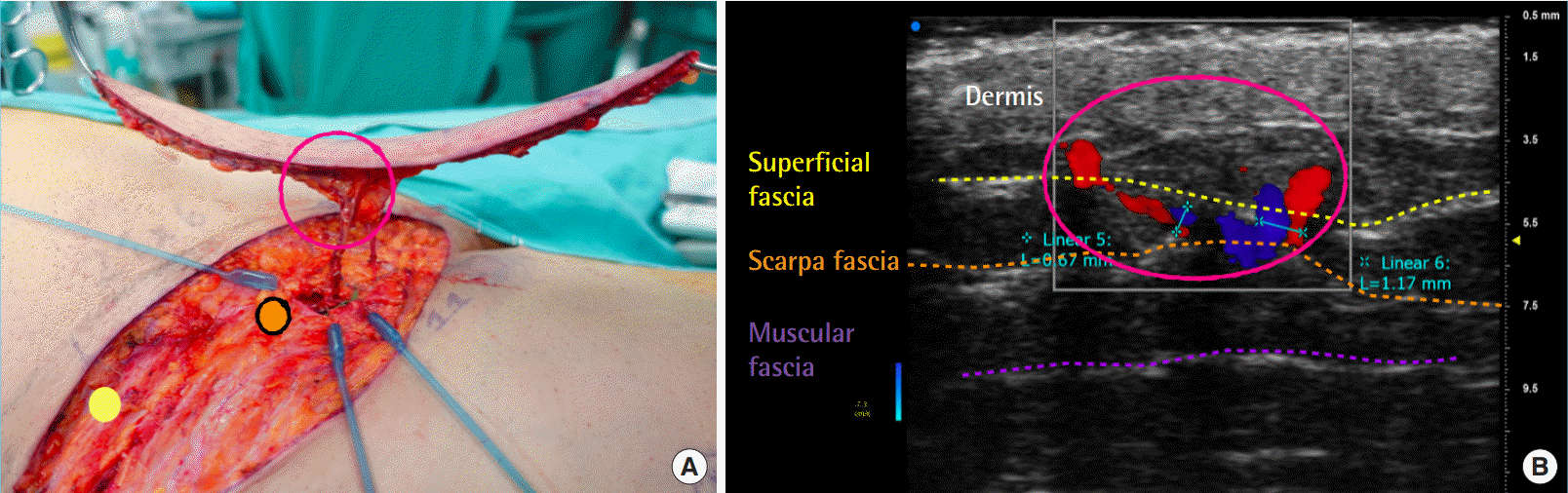
High-fidelity nature of UHF-US findings
Relationships between ultrahigh-frequency ultrasound (UHF-US) and intraoperative findings. (A) Intraoperative image of a superthin superficial circumflex iliac artery perforator flap. Yellow dot indicates superficial fascia, orange dot the Scarpa fascia. (B) High-fidelity microvascular planning using UHF-US. The caliber of the perforator was preserved from the muscular fascia to the superficial fascia. The yellow dotted line indicates the superficial fascia, and the orange dotted line denotes the Scarpa fascia. The pink circle indicates the high-fidelity agreement of the UHF-US findings with the intraoperative microvascular anatomy findings, both of which showed the main perforator piercing the superficial fascia to the level of the dermis and a small collateral vessel branching from the superficial fascia to the dermis.
All patients except one were of normal weight (body mass index, 18.5–24.9 kg/m2), while the remaining patient was obese (body mass index, 31.4 kg/m2). Patients’ mean age was 52 years (range, 47–68 years).
The superficial fascia had an average depth of 5.5 mm (range, 4–7 mm). The mean perforator caliber at the superficial fascial layer was 0.75 mm (range, 0.55–1.1 mm). In all cases, a clear slit was identified at the point at which the perforator pierced the superficial fascia. Of a total of 16 perforators studied, eight (four SCIP and four ALT) were classified as type 1 and eight (five SCIP and three ALT) as type 2. Based on this preoperative information, five flaps (four SCIP and one ALT) were harvested along the superficial fascial layer, whereas the remaining two flaps (both SCIP flaps) were elevated along the layer of the Scarpa fascia.
The mean flap size was 6.5 × 15 cm (range, 5 × 8 to 7.5 × 23 cm). Each flap was nourished by a single perforator. In all cases, the postoperative course was uneventful, and all flaps survived.
The final reconstructive outcomes were considered satisfactory by both the surgeon and the patients. The results included very good color matching, pleasant contour, good scar quality, and no limitation in range of motion, with an average follow-up time of 7 months (range, 2–15 months) (Fig. 4).
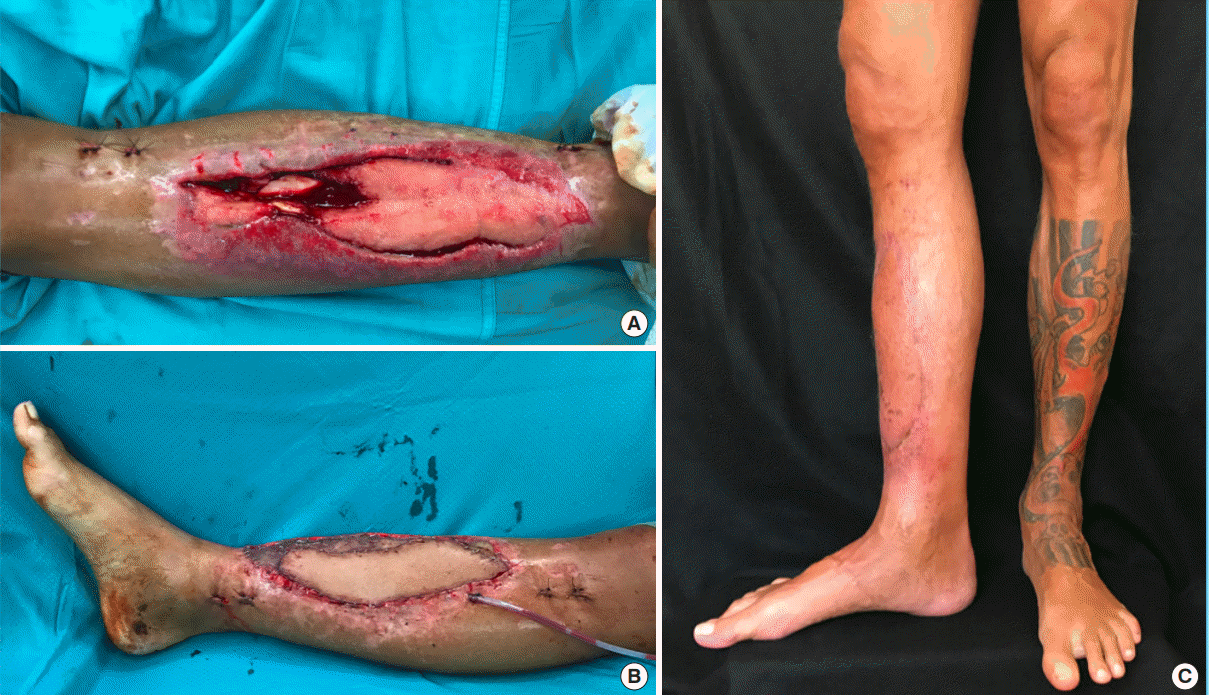
A case of lower limb resurfacing
A 42-year-old man presented with high-energy Gustilo IIIB trauma of the right lower limb. (A) Intraoperative image of the defect after internal fixation. (B) Final intraoperative result after endomedullary tibial nail and soft tissue reconstruction with a superthin anterolateral thigh flap revascularized to the posterior tibialis artery, one comitantes vein, and the great saphenous vein. The lateral leg compartment was covered with a split-thickness skin graft. (C) Three-month postoperative image showed complete functional recovery and a pleasant contour.
DISCUSSION
The terminology for thin flaps has often been used improperly, generating confusion. Thin flaps were initially described by Hong et al. [3], while superthin flaps were reported by Hyakusoku and Ogawa [4] as those thinned to the level where the subdermal vascular plexus can be seen through a minimal fat layer. Pure skin flaps, according to Narushima et al. [5], are those thinned to the level of the dermis. The anatomical features of these three different levels of thickness have been extensively described [8-11].
Since the 1990s, two main thinning techniques have been developed: (1) thinning after traditional harvest of an adipocutaneous flap by leaving a 3-cm cuff around the perforator or by using microdissection, and (2) direct thin flap elevation using the superficial fascia as the dissection plane [8].
Although direct elevation is now considered more efficient for homogenous thinning and less tedious than microdissection, the reported rate of partial or total failure of thin flaps is still high compared to that of traditional perforator flaps [6,7]. This may be due to the relative lack of microanatomical knowledge regarding these flaps. In recent years, an increased number of research studies have been published to broaden knowledge of the subcutaneous and microvascular anatomy of thin and pure skin perforator flaps [6,7,12-15].
In this paper, we propose a practical classification of perforators into two types based on their course within the adipose tissue, which can be easily identified preoperatively using UHF-US. We believe that this classification system can likely aid in flap design and donor-site choice and can enhance the safety of direct thin and superthin flap elevation.
UHF-US allows the visualization of microanatomical structures to the level of 30 μm, providing more detailed information regarding microvascular structures and subcutaneous anatomy than high-frequency US. A perforator can be followed to its entry point into the dermis and beyond, as previously reported [14,15].
According to the proposed classification system, a type 1 perforator is one for which a superthin flap (on the plane of the superficial/Camper fascia) can safely be directly harvested without necessitating microdissection, whereas a type 2 perforator is one for which a thin flap (on the plane of the Scarpa fascia) can similarly be directly harvested safely and without microdissection.
In conclusion, thin and superthin free flaps are becoming increasingly popular for improved functional and aesthetic defect reconstruction in thin areas. The microvascular anatomic features of such flaps are still under investigation, and no reliable methods have yet been proposed for precise preoperative planning. We found UHF-US to be very helpful in the preoperative planning of thin free and superthin flap harvest, as it enables a clear anticipation of very superficial microvascular anatomy, thereby facilitating safer, more precise, and faster direct elevation. UHF-US may represent the next frontier in preoperative planning associated with thin, superthin, and pure skin perforator flaps. We believe that the use of UHF-US requires ultrasonographic skills that should be first mastered using high-frequency US when planning to harvest perforator flaps.
Further research is needed to explore the maximal perforasome potential of such thin and superthin perforators.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Agostino Gemelli University Hospital (IRB No. ID#3051) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: G Visconti. Data curation: G Visconti, A Bianchi. Formal analysis: G Visconti. Methodology: G Visconti, A Cina. Project administration: G Visconti. Visualization: G Visconti. Writing - original draft: G Visconti. Writing - review & editing: G Visconti, A Hayashi, G Maccauro, G Almadori, M Salgarello.
Supplementary Material
Supplemental Video 1. Video showing the use of ultrahigh-frequency ultrasound in the preoperative planning of flaps with type 1 and type 2 perforators, providing insights regarding noteworthy ultrasound findings. Supplemental data can be found at: https://doi.org/10.5999/aps.2019.01179.v001
