Two international public platforms for the exposure of Archives of Plastic Surgery to worldwide researchers and surgeons: PubMed Central and Crossref
Article information

After changing the language of its articles from Korean or English to English only in 2012 [1], Archives of Plastic Surgery (APS) became an international journal, as evidenced by various metrics [2] and its inclusion in the Web of Science Core Collection in 2012 and Scopus in 2013. From 2018 to July 2020, authors from 45 countries published in APS (Supplemental Material 1). The 289 most recent articles from 2018 to present have been cited by researchers from 44 countries in articles in the Web of Science (Supplemental Material 2). These results originated from the APS editors’ laborious work on editing and publishing; furthermore, the content itself is top-tier in the field of plastic surgery. In this Editorial, I would like to explain the influence of two international public platforms that helped APS reach the international level through exposure to worldwide researchers and surgeons: PubMed Central (PMC) and Crossref. Of course, the influence of Google or Google Scholar on the exposure of APS to global researchers may have been greater than that of PubMed Central or Crossref. However, PubMed Central and Crossref are also powerful platforms for physicians and researchers.
MAINTENANCE AFTER BEING LISTED IN PMC
APS was accepted by PMC immediately after it became an English-only publication in 2012 [3]. Through 2014, English-only medical journals from Korea were accepted by PMC without difficulty. The PMC XML production quality of Korean publishing companies was excellent and followed the recommended tagging closely. However, starting in 2015, some journals from Korea began to be rejected from PMC due to scientific integrity and ethical issues. Furthermore, one journal from Korea was removed from PMC in 2018 for at least 2 years due to ethical issues. Even APS received a warning from PMC on ethical issues involving animal experiments and identifiable photographs in 2017, although these issues were resolved by publishing corrigenda [4]. Back in July 2006, I succeeded in producing PMC XML and converting it to HTML format to be shown on the website. I then taught this technique to the staff of the Korean Association of Medical Journal Editors and volunteers. After more than a year of discussions with PMC staff about XML quality, medical journals from Korea again began to be accepted by PMC starting on November 20, 2018. As of August 2020, the number of PMC journals from Korea was 129 (Supplemental Material 3). This number will continue to increase as numerous English-only journals re-apply to PMC despite initially being rejected.
What strategy should APS pursue to continue to be listed in PMC? In 2017, the warning from PMC was an excellent incentive to maintain the ethical standards of the journal [4]. Therefore, institutional review board approval, informed consent, and/or permission of the institutional animal care and use committee should be checked meticulously after receiving a manuscript that describes research on human or animal subjects. This is the editor’s or editorial assistant’s job. Checking the manuscript by a plagiarism checking program (Similarity Check/Crosscheck) is also mandatory before circulating a manuscript for peer review [5]. The above two tasks are all routine work by the editorial team, including that of APS, and will be able to eliminate some ethical misconduct. Continuing to be listed in PMC is crucial because it is the route for inclusion in PubMed, the most extensive medical literature database in the world, which has been maintained by the United States National Library of Medicine since June 26, 1997. There are two ways to be listed in PubMed: one is to be indexed in the MEDLINE database, and the other is to be listed in PMC, which automatically transfers its metadata to PubMed. Medical editors in Korea planned the strategy to be listed in PMC first because being indexed in MEDLINE was known to be a difficult task, with an acceptance rate by the Literature Selection Technical Review Committee (LSTRC) of 10% to 15% every year. Medical editors, therefore, sought to find a way to bypass review by the LSTRC to be listed in PubMed. This strategy was successful through 2014. Starting in 2015, the review process by PMC was strengthened, which caused some medical journals from Korea to be rejected as PMC journals. Currently, the level of review for scientific integrity and ethical standards is believed to be almost the same between LSTRC and PMC reviewers. The LSTRC considers importance to readers as well as content quality and ethics policies [6,7]. Because the ethical policies of APS are excellent and its practices are carefully adhered to for each article, it is time for APS to apply to MEDLINE again. To do so, it will be necessary to clarify the importance of APS to researchers, clinicians in the field, clinicians not in the field, educators, administrators, allied health professionals, students, and even policymakers. Furthermore, examples of articles relevant to each category of readers should be provided. Being indexed in MEDLINE will be another way to confirm the journal’s scientific integrity, excellent ethical standards, and a variety of readership. The LSTRC only considers article content itself and does not count citation frequency, unlike the reviewers and editors for Scopus or Science Citation Indexing Expanded.
SITTING ON THE SHOULDERS OF CROSSREF
Some Korean journals published by international commercial companies started to be equipped with digital object identifiers (DOIs) before 2007. In September 2007, society journals in Korea, which are published through collaboration with local publishing companies, began to add DOIs to their articles. The first society journal in Korea with DOI descriptions was the Journal of Korean Ophthalmological Society. Medical journals have subsequently been leaders in adopting Crossref’s services. Producing Crossref XML for depositing DOIs was easier than PMC XML production because only metadata information was necessary at that time [8]. As Crossref was founded in 2000, 7 years passed before society journals in Korea introduced this standard for providing a continuous link to full-text articles [9]. The turning point of Korean journals in adopting Crossref’s services was the policy of the Korean Federation of Science and Technology Societies (KOFST) in 2011 that asked all scientific journal editors to add DOIs to their articles. KOFST indicated that it would no longer provide financial support for journals if articles had no DOIs, because DOIs are a minimum requirement for a scholarly journal. Therefore, all scientific journals, including medical journals, started to add DOIs and to make DOI landing pages. This is the reason why the number of journals with DOIs increased from 256 (47.1%) in 2011 to 536 (98.5%) in 2019 [10].
APS rapidly adopted Crossref services after the inauguration of its new title with English-only full-text articles in 2012, as follows: DOI, Crossmark (check for updates), Fundref (Funder Registry), Text and Data Mining, Crosscheck (Similarity Check), Reference linking, Cited-by, and the Metadata Retrieval Representational State Transfer (REST) application programming interface (API) [11]. The metrics service is reflected on the journal homepage through the Crossref metadata REST API, as can be seen at https://www.e-aps.org/ (Fig. 1). APS is a role model for plastic surgery journals throughout the world in terms of adopting Crossref services and reflecting them on the journal homepage. Therefore, it is easy to follow information to and from APS while web-surfing and to identify the present position of the journal in the world journal market.
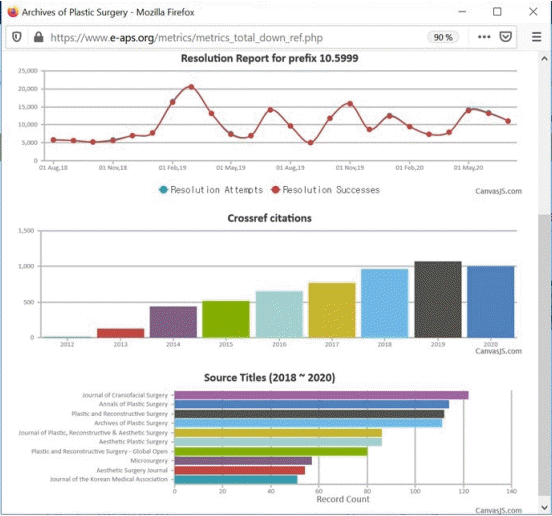
Metrics functions on the journal homepage
Screenshot of the metrics functions on the journal homepage of Archives of Plastic Surgery (cited August 24, 2020).
Crossref’s devotion to nonprofit organization journals has been enormous. Without Crossref services, nonprofit societies could not compete with commercial publishing companies in the journal network market. The infrastructure of Crossref has provided an ideal environment for society journals. In a recent survey by Crossref, there was a concern, “which came from longer-term members of the organization, who were worried that Crossref had become distracted from serving their needs, and was focused on new publishing models and the long-tail of members” [9]. Such criticism may reflect worries about the concentration of Crossref’s services for small society journals. I am worried about the withdrawal of large commercial companies from Crossref because they are the main supporters of Crossref through their substantial membership fees. Their devotion to Crossref created a new platform for local society journals like APS in Korea. These journals have been published through the collaboration of academic societies with local publishing companies with separate functions for manuscript editing, English proofreading, an e-submission system, XML and homepage production, and illustrations. Without Crossref, local society journals might not be able to survive in the journal market. Therefore, they should establish a new platform like Crossref for the above functions. I hope that the interests of longer-term members and the local long-tail of members can converge into the same goal—devotion to world citizens’ health and happiness and cultural development by publishing scientifically relevant articles—through Crossref’s services.
New Crossref services will appear, including data citation [12], the Research Organization Registry [13], and Participation Reports [14]. Data citation is officially described as follows: “the data citation is done when a journal article references the data the research was built upon or references, in the same way, that a paper would normally provide a reference list to other scholarly resources, such as other papers” [12]. Therefore, publishers should announce a data policy and data citation method. APS has already encouraged data sharing in its instructions to authors (https://www.e-aps.org/authors/authors.php) wherever possible, unless this is prevented by ethical, privacy, or confidentiality matters. However, this only reflects an encouragement to share data. There are not many articles with data sharing in APS. Only one article published in 2015 was found by searching for Associate Data in PubMed [15]. APS also follows the “Data Sharing Statements for Clinical Trials: A Requirement of the International Committee of Medical Journal Editors.” Therefore, articles describing clinical trials such as randomized controlled studies, pre- and post-paired comparison clinical studies, and cohort studies should disclose a data sharing statement. Since 2018, there has been a lack of clinical trial studies. Only one cohort study was found [16]. However, that study was started in 2010, when clinical trial registration was not mandatory. Although it is not compulsory, it may be preferable to present a data sharing statement as suggested by Jeong (2020) [17], using one of the following examples:
“Example 1. The clinical trial data of this article will not be shared.
Example 2. The clinical trial data of this article are available upon reasonable request to the corresponding author.
Example 3. All of the individual participant data collected are available from a data repository immediately after publication without an end date. The study protocol, statistical analysis plan, informed consent form, clinical study report, and analytic code are also available. Anyone can access the data, and the data can be used for any purpose.”
Data sharing is not mandatory, although the journal follows the Data Sharing Statements for Clinical Trials. It is sufficient to ask authors to provide a data sharing statement as they deem appropriate, which means that the authors’ statement itself is necessary. Still, a few journals in Korea have maintained clinical trial data sharing policy [17,18]. If data are deposited in a repository, such as Harvard Dataverse [19], a DOI for the data is automatically generated when data are deposited. It is necessary to cite the corresponding DOI in the statement of data availability. More detailed instructions on how to adopt data sharing were explained in the previous Editorial [20].
The Research Organization Registry is a unique identifier of each organization or institute [13]. The name of the organization can be changed, and organizations may merge or be separated. This registry is the way to maintain an organization’s uniqueness. If this initiative is implemented, the unique ID of the organization should be added to the metadata tag. Guidance on standard practices may be published.
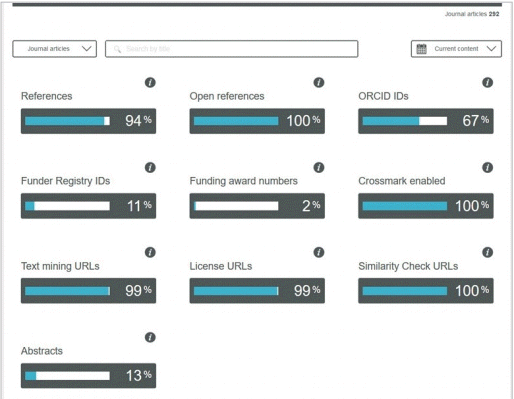
Participation Reports are a visualization of the metadata available through Crossref’s public REST API [14]. It is still a beta service. Fig. 2 shows the percentage of APS deposits registered for each metadata element. The deposit rate of the abstracts element was 13%. It is required to add abstracts and to re-deposit the metadata to improve this rate. The deposit rate of the ORCID (Open Researcher and Contributor ID) element was 67%. Some articles provide all co-authors’ ORCID IDs; while others provide ORCID IDs of some of the co-authors. By asking all coauthors to provide their ORCID IDs, it will be possible to increase this rate to 100%. The rate mismatch between Funder Registry IDs and funding award numbers originates from the omission of the funding award number in the description of funding. For example, the following is the case of neglecting to provide a grant number [21]: “This study was supported in part by the Mayo Clinic Center for Individualized Medicine, and the Plastic Surgery Foundation.” Presumably, the grant number of the institute was not provided; otherwise, it was omitted during submission. Therefore, authors should be recommended to provide the grant number.
MAINTENANCE OF JOURNAL QUALITY AND EARLY ADOPTION OF NEW CROSSREF SERVICES
To continue to be listed in PMC and PubMed, the journal quality should be maintained. Consistent support for editors and the editorial team is essential to maintain the present quality level, which is already top-tier. Constant monitoring and immediately adopting Crossref’s new services is also a good method for a local society journal to keep up with evolving standards of scholarly journal publishing. The issues mentioned above should be revised or upgraded as a matter of editorial policy, and the cooperation with engineers is necessary to improve the Crossref Participation Report.
Notes
Conflict of interest
SH has been an ethics editor since 2012. He was not involved in the peer reviewer selection, evaluation, or decision process of this editorial. No other potential conflicts of interest relevant to this editorial were reported.
Supplementary Material
Supplemental Material 1.
Countries of authors of articles published in Archives of Plastic Surgery from 2018 to July 2020. Supplemental data can be found at: https://doi.org/10.5999/aps.2020.01697.S001.
Supplemental Material 2.
Countries of authors citing articles published in Archives of Plastic Surgery from 2018 to July 2020 from the Web of Science Core Collection. Supplemental data can be found at: https://doi.org/10.5999/aps.2020.01697.S002.
Supplemental Material 3.
NLM catalog showing PMC journals published in Korea (cited 2020 August 24, 2020) available from: https://www.ncbi.nlm.nih.gov/nlmcatalog/. Supplemental data can be found at: https://doi.org/10.5999/aps.2020.01697.S003.
Supplementary materials are available from Harvard Dataverse: https://doi.org/10.7910/DVN/YSUBB5.