Intraoperative blood loss and surgical time according to the direction of maxillary movement
Article information
Abstract
Background
Excessive bleeding is one of the most severe complications of orthognathic surgery (OGS). This study investigated the associations of intraoperative blood loss and surgical time with the direction of maxillary movement.
Methods
This retrospective study involved patients who underwent OGS from October 2017 to February 2020. They were classified based on whether maxillary setback was performed into groups A1 and B1, respectively. Relative blood loss (RBL, %) was used as an indicator to compare intraoperative blood loss between the two groups. The surgical time of the two groups was also measured. Subsequently, the patients were reclassified based on whether posterior impaction of the maxilla was performed into groups A2 and B2, respectively. RBL and surgical time were measured in the two groups. Simple linear and multiple regression analyses were performed. P-values <0.05 were considered to indicate statistical significance.
Results
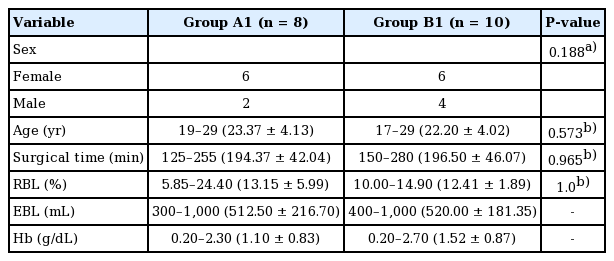
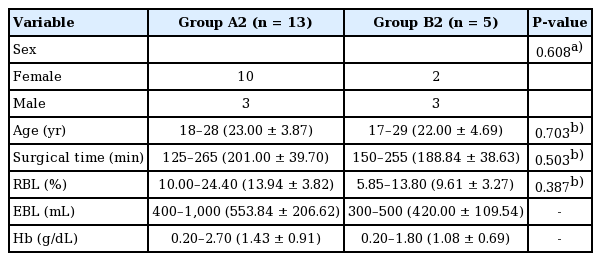
Eighteen patients were included. The RBL and surgical time for the groups were: A1, 13.15%±5.99% and 194.37±42.04 minutes; B1, 12.41%±1.89% and 196.50±46.07 minutes; A2, 13.94%±3.82% and 201.00±39.70 minutes; and B2, 9.61%±3.27% and 188.84±38.63 minutes, respectively. Only RBL showed a statistically significant difference between the two groups (A2 and B2, P=0.04).
Conclusions
Unlike maxillary setback, posterior impaction of the maxilla showed a significant association with RBL during surgery. When performing posterior impaction of the maxilla, clinicians need to pay particular attention to surgery and postoperative care.
INTRODUCTION
Orthognathic surgery (OGS) involves repositioning of the maxilla and mandible to an ideal configuration. If the maxilla and mandible are not in an ideal position, problems with occlusion, mastication, pronunciation, and facial appearance may occur. After Le Fort I osteotomy and bilateral sagittal split ramus osteotomy, the maxilla and mandible can be moved in any direction depending on the purpose of OGS. In the past, OGS was mainly aimed at functional improvements, such as improvement in occlusion, and advancement of the maxilla and mandibular setback were predominant in Korea. Recently, however, dual advancement of the maxilla and mandible has been attempted to improve the function of the upper airway in patients with conditions such as obstructive sleep apnea. In addition, as the aesthetic needs of patients have increased, it has become more common to perform simultaneous setback of the maxilla and mandible, as well as posterior impaction of the maxilla for more mandibular setback.
In previous studies, OGS was considered to be a safe surgical procedure with few complications [1,2]. However, other studies have suggested that some complications may occur, such as malunion of the bone, temporomandibular joint (TMJ) disorders, nerve injury, and excessive blood loss [2,3]. Excessive blood loss is one of the most severe complications [3]. In addition to hemodynamic instability, excessive bleeding can cause direct airway compromise arising from blood clots and indirect airway problems caused by deviation or compression of the airway by a hematoma [4]. Therefore, the quantity of blood lost is the most important factor associated with the risk of OGS. Although advanced surgical techniques can reduce blood loss during OGS, the risk of excessive blood loss cannot be overlooked owing to the complex vascular anatomy of the maxilla and the difficulty of visualizing the surgical field. In maxillary setback and posterior impaction of the maxilla through Le Fort I osteotomy, a large amount of bleeding is inevitable due to the abundant pterygomaxillary vascular network [5]. Therefore, in surgical procedures for managing the pterygoid process and the posterior segment of the maxilla, it is vitally important to take sufficient time to avoid unintended bleeding.
The purpose of this study was to compare intraoperative blood loss and surgical time in patients who underwent OGS with or without maxillary setback, and with or without posterior impaction of the maxilla.
METHODS
This retrospective study included patients who underwent OGS from October 2017 to February 2020 at a craniofacial surgery center. Patients who underwent mandibular or maxillary one-jaw OGS and anterior segmental surgery were excluded. Patients who underwent concomitant facial contouring surgery, except genioplasty, were also excluded. All patients were screened before surgery for contraindications of hypotensive anesthesia, such as ischemic heart disease, hypertension, peripheral vascular disease, and cerebrovascular disease [6].
Information on the following parameters was collected for data analysis; (1) patient demographics (age, sex, body weight, and diagnosis); (2) surgical indices (type of surgical procedure, surgical time, intraoperative estimated blood loss [EBL], relative blood loss [RBL], major vascular injury during surgery, planned length of maxillary setback, planned length of maxillary posterior impaction); or (3) laboratory indices (coagulation value [prothrombin time], preoperative and postoperative hemoglobin level).
The patients were initially divided into two groups, A1 and B1, according to whether they underwent maxillary setback. Subsequently, patients were divided into two groups, A2 and B2, depending on whether they underwent maxillary posterior impaction (moving the molar teeth–bearing posterior portion of the maxilla upward).
Surgical time and RBL were measured. Surgical time was measured from the injection of local anesthetics to the closure of all wounds. RBL, which is obtained by dividing EBL by the estimated blood volume (EBV), represents blood loss as a percentage and takes the patient’s weight into consideration. Patient-specific RBL was calculated as the EBL divided by the EBV.
The amount of EBL was determined by subtracting the amount of saline irrigation solution used during surgery from the volume of the suction bottle. In principle, the estimated value of the intraoperative blood-soaked gauze should be added; however, this was negligible, and it was not considered in the calculation. Patients’ body weight was measured in the hospital ward on the day before surgery. The EBV was determined using the weight calculation method (reference: 75 mL/kg for men and 65 mL/kg for women) [7].
Surgical technique
All operations were performed by a single surgeon (JHC). In all cases, the surgeon did thorough presurgical planning in consultation with experienced orthodontists. Dissection began at the maxilla. A mucosal incision was made and subperiosteal dissection around the pyriform aperture was performed using electrocautery to minimize bleeding during incision and elevation. This was followed by subperiosteal elevation. After full elevation and protection of the maxillary periosteum, Le Fort I osteotomy was performed with a reciprocating saw and a straight osteotome. Immediately after an osteotomy line was made, the tip of the electrocautery device was advanced between the gap in the osteotomy line, and hemostasis of the sinus lining was performed. After a down-fracture of the maxilla, tight gauze packing at the posterior wall of the maxilla was done for hemostasis. The focus was moved to the mandible, and subperiosteal dissection was performed to stop maxillary bleeding (Fig. 1). After mandibular dissection and gauze packing, the focus was returned to the maxilla. After the completion of maxillary fixation, the focus was moved back to the mandible and osteotomy and fixation of the mandible were performed. The techniques for maxillary setback and posterior impaction are described in greater detail in the Discussion section of this paper. Patients were admitted to the intensive care unit after extubation on the day of surgery for thorough postoperative management.
Data analysis
The data were analyzed using SPSS for Windows version 21.0 (IBM Corp., Armonk, NY, USA).
In the primary analysis, variables were compared between the two groups to identify confounding variables. Differences in the surgical time and RBL between the two groups were analyzed using the Mann-Whitney test between unpaired samples, because the distribution showed non-normality and the sample size was less than 30. Next, simple linear regression analysis was performed for RBL and the surgical time to determine their associations with the variables. In addition, a statistical analysis was performed to exclude the effects of types of surgical procedures on intraoperative bleeding and surgical time. A simple linear regression analysis for RBL and surgical time were performed according to whether genioplasty was performed. Multiple regression analysis was used to determine whether patients’ demographic data and treatment groups were significantly associated with RBL and surgical time during OGS. P-values <0.05 were considered to indicate statistical significance.
This study was conducted in accordance with the principles of the Declaration of Helsinki. All patients were provided with a description of the study before surgery, and informed consent was obtained for inclusion. The institutional review board of our institution reviewed the protocol and approved this study (IRB No. MJH2020-01-007).
RESULTS
Eighteen patients were recruited into the study based on the patient selection criteria. The number of patients included in each group was as follows: eight in A1, 10 in B1, 13 in A2, and five in B2. Patients ranged in age from 17 to 29 years (mean, 22.72±3.88 years). Six patients (33%) were men and 12 (66%) were women.
All patients had normal coagulation values (prothrombin time, %). The distribution of the characteristics stratified by group is presented in Tables 1 and 2. The planned length of the maxillary setback and the posterior impaction of the maxilla were 0.3 to 5.0 mm (mean, 2.13±1.84 mm) and 2.5 to 5.0 mm (mean, 4.03±0.82 mm), respectively. Patients with maxillary impaction only underwent two procedures: posterior impaction or total impaction.
No statistically significant differences were observed in the variables analyzed in this study between A1 and B1 or between A2 and B2. This suggests that the initial characteristics of patients did not influence between-group differences in outcomes. Therefore, it was considered appropriate to compare the postoperative results of the two groups (Tables 1, 2).
The specific diagnoses of the patients were as follows: three patients had congenital dentofacial deformities (17%; secondary deformity of cleft lip and palate, n=2; Crouzon syndrome, n=1), one patient had obstructive sleep apnea (5.5%), and the remaining patients had acquired dentofacial deformities (e.g., prognathism), facial asymmetry, or no malocclusion (i.e., the procedure was done for purely aesthetic purposes) (77.5%).
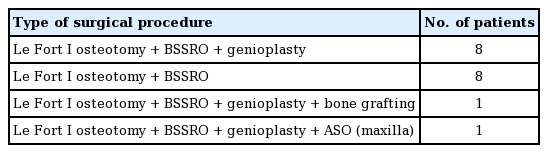
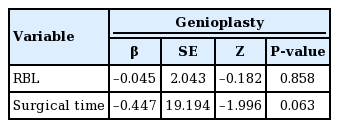
The types of surgical procedures performed on the patients are presented in Table 3. Genioplasty was not found to have statistically significant relationships with RBL or surgical time (P=0.063 for surgical time, P=0.858 for RBL) (Table 4).
There were no major vascular injuries during surgery in any of the patients in this study.
Groups A1 and B1 (based on whether maxillary setback was performed)
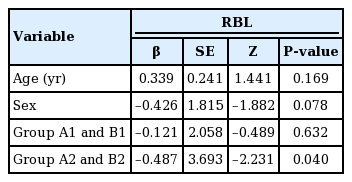
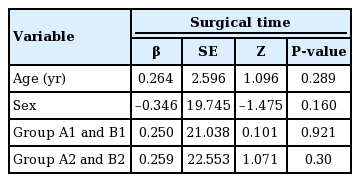
No significant associations between RBL and age, sex, and treatment group were revealed by the simple regression analysis. Similarly, there were no significant associations between the surgical time and age, sex, or treatment group (Tables 5, 6).
Groups A2 and B2 (based on whether posterior impaction of the maxilla was performed)
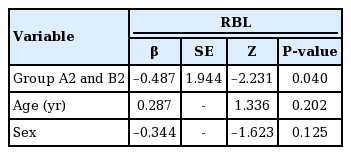
No statistically significant difference was found between groups A2 and B2 in terms of surgical time; however, RBL differed between these two groups (Tables 5, 6). Multivariate regression analysis demonstrated a significant association between RBL and treatment group (P<0.05 for RBL) (Table 7).
DISCUSSION
OGS can correct severe malocclusions that cannot be cured by orthodontic treatment alone [8]. For this reason, its purpose was thought to be more functional than aesthetic. However, malocclusion is one of many symptoms and signs that can occur when the maxilla and mandible are not in a good position. Facial disfigurement is not a negligible consideration; sometimes, facial appearance is the most important concern for patients [9]. Malocclusion results from malposition of the jawbones, which can disappear after repositioning; therefore, orthodontic treatment is required. Thus, Rosen reported that OGS must be considered to be aesthetic surgery, with normal occlusion as an extra benefit [10]. Other studies, however, concluded that appearance is part of the function of the face [11]. Thus, we consider OGS to be functional aesthetic surgery.
OGS has become one of the most popular aesthetic procedures in Korea. As it has become widely performed in many private clinics, many complications have occurred and received considerable media attention. To date, no investigations have been conducted and no formal reports have been published on the causes of the most severe complications after OGS performed in private clinics. However, it is thought that frequent causes of complications include hypovolemic shock from massive bleeding and airway problems. Airway problems include direct compromise caused by blood clots and indirect compression by hematoma [4]. The outcomes of bleeding cause problems, making it important to strive to reduce the amount of bleeding during surgery. It is equally important to predict and prepare for intraoperative blood loss based on the type of surgical technique to be performed.
Most previous studies have reported that OGS rarely leads to complications and has a low transfusion potential in OGS [1-3,12,13]. Nevertheless, every surgical procedure poses a risk of complications, including OGS. Possible complications of OGS include excessive bleeding, infection, nerve injury, TMJ problems, infection, necrosis of bony segments, hearing problems, vision impairment, and neuropsychiatric problems [1-3,14]. Excessive bleeding caused by vascular injury can be fatal if hemodynamic instability occurs [3]. Airway compromise at the floor of the mouth, hematoma, and edema have been reported in rare cases [2,4,15]. According to a retrospective study by van de Perre et al. [16] that involved 2,049 patients who underwent OGS, excessive blood loss was the most frequent complication.
For this reason, clinicians have made efforts to reduce intraoperative bleeding in OGS. Previous studies have revealed several factors that can reduce blood loss during OGS, including the duration of surgery, type of surgical procedure, proficiency of the surgeon, and type of anesthesia [2,17-22]. Additionally, in 1999, Rohling et al. [23] reported that methods for reducing intraoperative bleeding during OGS included the use of an atraumatic surgical approach, moderate hypotension control, and placement of the surgical site above the heart.
The most important requirement for a surgeon to reduce blood loss during OGS is proficiency in performing surgical procedures without inducing major vascular injury. Although advanced surgical techniques can reduce blood loss during OGS, the risk of excessive blood loss cannot be overlooked because of the complex vascular anatomy of the maxilla and mandible, and the difficulty in visualizing the surgical field [5].
This study compared the amount of intraoperative bleeding and the surgical time in patients who underwent OGS with or without maxillary setback, and with or without posterior impaction of the maxilla. RBL was significantly different between groups stratified according to whether posterior impaction of the maxilla was performed. In the group with posterior impaction of the maxilla, RBL was significantly higher, but no significant difference in surgical time was found. RBL and surgical time were not significantly different between the groups stratified by whether maxillary setback was performed.
In several previous studies, decreased hemoglobin levels after surgery were considered to be an indicator of intraoperative blood loss [21,24]. However, several variables affect hemoglobin levels, undermining its use as an indicator of blood loss. The timing of hematologic studies before and after surgery varies from patient to patient, and hemoglobin levels are also affected by the patient’s plasma volume; the hemoglobin level may be low when patients are hydrated and high when they are dehydrated [25]. Several studies have also investigated intraoperative blood loss in terms of absolute blood loss, rather than RBL, which is a measure that considers individual patients’ weight. Recently, a study was conducted on RBL, and confirmed that a benefit of this method is that accurate measurements can be made without being affected by the patient’s weight [13].
No statistically significant difference was found in RBL and surgical time between the groups defined according to whether maxillary setback was performed. This may be attributed to various factors, but the authors postulate that the surgical technique was a major factor. When the authors returned to the maxilla during procedures after subperiosteal elevation of the mandible, the amount of bleeding in the maxilla was negligible. To secure space for maxillary setback, the medial and lateral pterygoid plates, which interfered with the posterior wall of the maxilla, were fractured and retracted together. First, subperiosteal dissection of the medial surface of the medial pterygoid plate and the lateral surface of the lateral pterygoid was performed, followed by an incomplete fracture with a small burr and osteotome. Subsequently, the two tips of a large rongeur were introduced into the subperiosteal dissection space. A gentle twist of the rongeur was sufficient to accomplish the pterygoid plate fracture for maxillary setback. In the course of the procedure, venous bleeding from the pterygoid venous plexus occurred, but the amount was minimal and the bleeding ceased shortly with Surgicel packing (Johnson & Johnson, New Brunswick, NJ, USA). Compression by the retracted maxilla was thought to have a positive effect on hemostasis (Fig. 2). However, this finding differs from the results of previous studies, which showed longer surgical time and higher intraoperative blood loss in the maxillary setback group [26]. Mean RBL was higher in group A1, but this difference was not statistically significant, possibly because of the small sample size of our study.
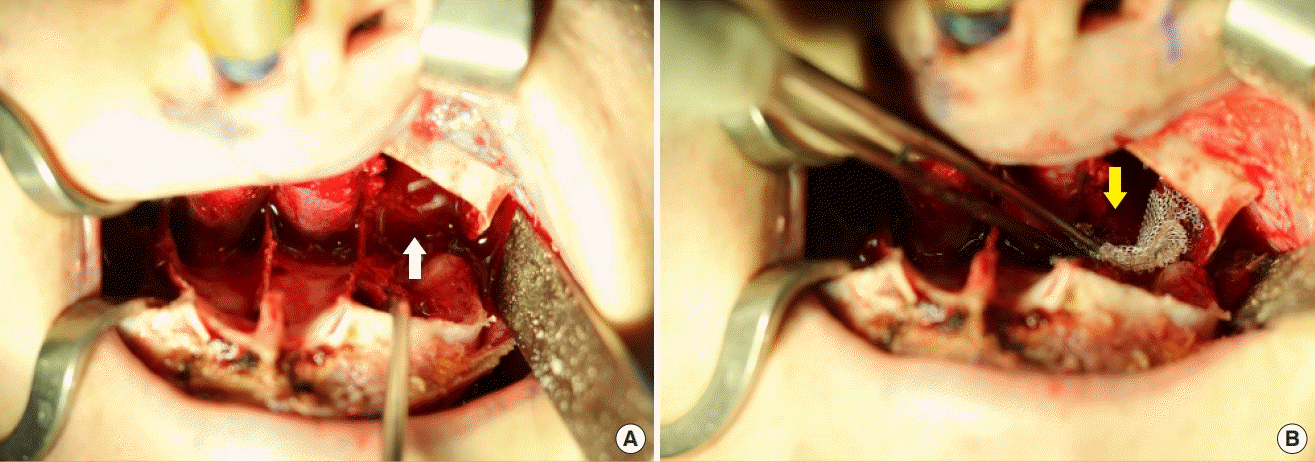
Venous bleeding from pterygoid venous plexus
(A) Pterygoid venous plexus bleeding after pterygoid plate fracture (white arrow). (B) Tight gauze packing and Surgicel were useful for hemostasis (yellow arrow).
For posterior impaction of the maxilla, trimming of the bony canal around the descending palatine artery was required. Although there was no statistically significant difference in the surgical time between groups in our study, the procedures lasted approximately 10 to 20 minutes. After removal of the packed gauze at the posterior wall of the maxilla, the bony canal around the descending palatine artery was removed using a small rongeur and a burr. No direct vessel injuries occurred in any cases in this study, but it took approximately 10 to 30 minutes to perform bony canal trimming on both sides. Even without maxillary setback, fracturing the pterygoid plate was inevitable due to interference with the maxillary tuberosity or maxillary posterior wall, and bleeding due to the pterygoid plate fracture could be controlled through rapid hemostasis using Surgicel (Fig. 2B).
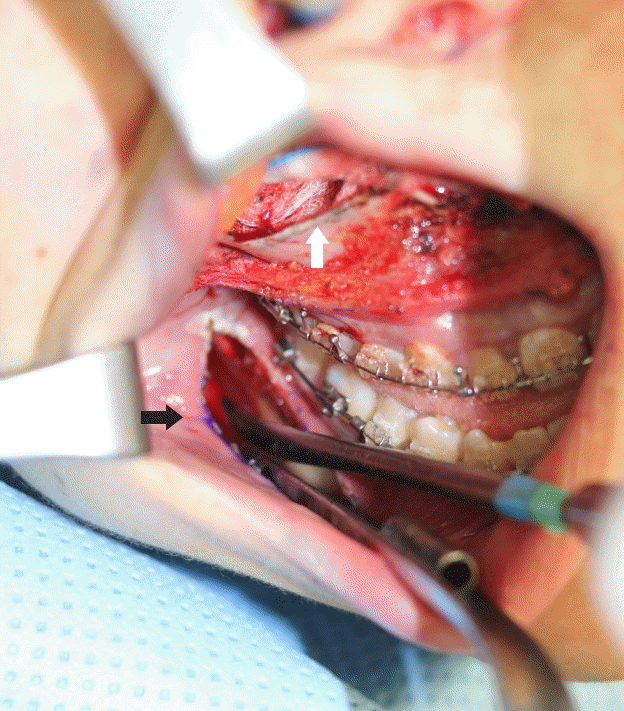
The only difference between the maxillary setback and posterior impaction procedures was trimming of the bony canal around the descending palatine artery for approximately 10 to 20 minutes. During this time, the minor bleeding of the posterior wall of the maxilla was not enough to conduct hemostasis without a specific focus, and the area slowly filled up. Thus, surgery was performed with suction when blood obstructed the surgical field (Fig. 3). During this process, the amount of suctioned blood could not be measured separately; the bleeding was not active. However, the amount of blood suctioned during the 10 to 30 minutes of bony canal trimming may have contributed to the statistically significant difference between groups A2 and B2.
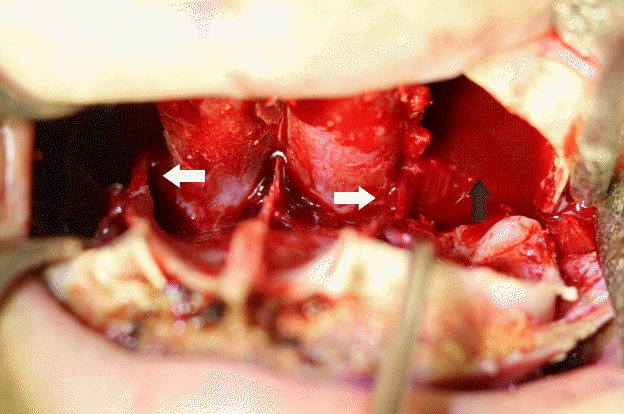
Trimming bony canal around descending palatine artery
There were no major vascular injuries in any of the patients in this study. We routinely attempted to preserve the descending palatine neurovascular bundle (white arrows). Minor bleeding of the posterior wall of the maxilla caused the area to fill up slowly (black arrow).
When performing OGS in patients with a secondary deformity of cleft lip and palate, mucoperiosteal dissection at the maxilla takes longer than in normal patients due to soft tissue scarring of the maxilla. In our study, there was no significant difference in surgical time between groups A2 and B2, which were stratified by the performance of posterior impaction of the maxilla. This may be attributed to the small size of the B2 group (n=5) and the inclusion of two patients (40%) with a secondary deformity of cleft lip and palate.
Most patients undergo OGS surgery to improve their appearance; thus, they are very sensitive to postoperative complications and the time that will be necessary to return to their daily life. Medical disputes are becoming more common, and medical knowledge is increasingly accessible; hence, patients’ medical knowledge is increasing.
Based on the results of this study, the authors propose that clinicians consider the influence of surgical procedures on RBL and surgical time. In patients with posterior impaction of the maxilla, caution should be exercised and postoperative management should be performed.
This study has some possible limitations. First, because each patient group had fewer than 30 cases—reflecting a smaller sample than other studies—the results of this study may not be generalizable. Second, the average age of the patients was 22.7 years old; overall, the patients were relatively young. Most of the patients (77.5%, n=15) had surgery for aesthetic purposes, which may have accounted for this age distribution. Although the impact of this issue on the research results is unclear, it may be a limitation.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Myongji Hospital (IRB No. MJH2020-01-007) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: HS Kim, JH Son, JH Chung, KS Kim, J Choi, JY Yang. Data curation: HS Kim, JH Son. Formal analysis: HS Kim, JH Son. Methodology: HS Kim, JH Son. Project administration: HS Kim, JH Son. Visualization: HS Kim, JH Son. Writing - original draft: HS Kim, JH Son, JH Chung. Writing - review & editing: HS Kim, JH Son, JH Chung, KS Kim, J Choi, JY Yang.