Blossom smart expander technology for tissue expander-based breast reconstruction facilitates shorter duration to full expansion: A pilot study
Article information
Abstract
Background
This study evaluated the Blossom system, an innovative self-filling, rate-controlled, pressure-responsive saline tissue expander (TE) system. We investigated the feasibility of utilizing this technology to facilitate implant-based and combined flap with implant-based breast reconstruction in comparison to conventional tissue expansion.
Methods
In this prospective, single-center, single-surgeon pilot study, participants underwent either implant-based breast reconstruction or a combination of autologous flap and implantbased breast reconstruction. Outcome measures included time to full expansion, complications, total expansion volume, and pain scores.
Results
Fourteen patients (TEs; n=22), were included in this study. The mean time to full expansion was 13.4 days (standard error of the mean [SEM], 1.3 days) in the combination group and 11.7 days (SEM, 1.4 days) in the implant group (P=0.78). The overall major complication rate was 4.5% (n=1). No statistically significant differences were found in the complication rate between the combination group and the implant group. The maximum patient-reported pain scores during the expansion process were low, but were significantly higher in the combination group (mean, 2.00±0.09) than in the implant group (mean, 0.29±0.25; P=0.005).
Conclusions
The reported average duration for conventional subcutaneous TE expansion is 79.4 days, but this pilot study using the Blossom system achieved an average expansion duration of less than 14 days in both groups. The Blossom system may accommodate single-stage breast reconstruction. The overall complication rate of this study was 4.5%, which is promising compared to the reported complication rates of two-stage breast reconstruction with TEs (20%–45%).
INTRODUCTION
The American Society of Plastic Surgeons reported a total of 101,657 breast reconstruction cases in 2018. Tissue expansion with implants continues to be the most popular modality for breast reconstruction. Despite a 1.5% decrease from the year prior, tissue expansion with implants constituted 68.8% of breast reconstruction cases in 2018 [1].
In 1982, Radovan [2] presented his experiences with 68 patients utilizing a two-stage prosthetic breast reconstruction technique with saline tissue expanders (TEs) followed by implant exchange. Almost 40 years later, multiple innovations have emerged aiming to circumvent the frequent postoperative visits and lengthy expansion period that Radovan’s technique necessitated, including self-filling osmotic expanders by Austad and Rose [3], patient-controlled carbon dioxide-filled expanders by Han et al. [4], and a patient-controlled expansion device by Widgerow et al. [5]. Despite researchers’ efforts, these alternative approaches have not entered widespread use and breast reconstruction with conventional tissue expansion persists relatively unchanged since its inception.
Conventional tissue expansion begins with TE insertion into the dissected breast pocket after mastectomy. The TE is then filled through an incorporated or remote port through percutaneous saline boluses serially injected at 1- to 3-week intervals following a 2- to 3-week postoperative period to allow for primary healing of the incisions. Volume expansion through conventional TEs requires an average of 8–12 weeks to complete [6]. The TE is then exchanged for a definitive implant, traditionally at 6 months from full expansion.
This study aimed to evaluate an innovative self-filling, rate-controlled, pressure-responsive saline TE system with an external sensing device. We investigated the feasibility of utilizing this technology to facilitate implant-based and flap with implantbased breast reconstruction in comparison to conventional tissue expansion.
METHODS
The Blossom Smart Expander
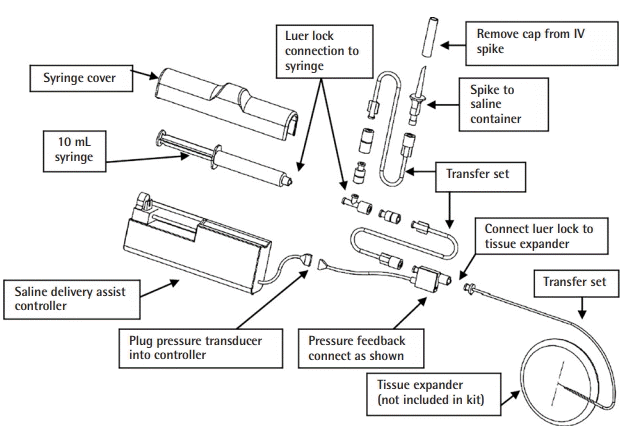
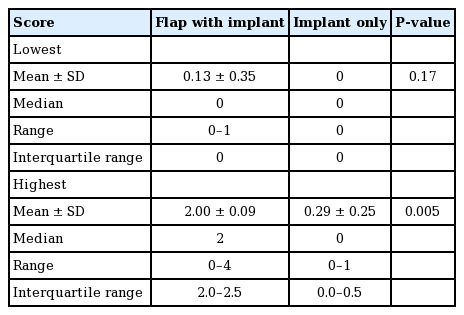
The Blossom Smart Expander technology (Marz Medical Inc., Mountain View, CA, USA), is a commercially available system that delivers small volumes of sterile saline from an external pouch through a controller with programmable pressure-responsive flow profiles (Figs. 1, 2). The Blossom system contains a pressure sensor, and once this reading exceeds the programmed maximum, saline delivery is halted. Saline delivery resumes after pressure readings fall below the programmed threshold. The Blossom system and TE are inserted either in a delayed fashion following mastectomy or immediately following mastectomy. Removal of the Blossom system, along with the port and fill tubing, may be completed in a clinical setting without local anesthesia.
The Blossom tissue expander system
The Blossom tissue expander system includes the controller housing a 10-mL syringe and automatic pump mechanism with pressure feedback through the transducer. This technology is used with a remote port expander (e.g., Mentor Spectrum adjustable saline implant). The controller is adapted for substantially continuous operation in response to pressure within the tissue expander. The controller is able to deliver saline to the expander when the pressure within the expander is below a predetermined threshold, while withholding delivery of the fluid when the pressure is above the set threshold or reaches a predetermined maximum volume.
Study population
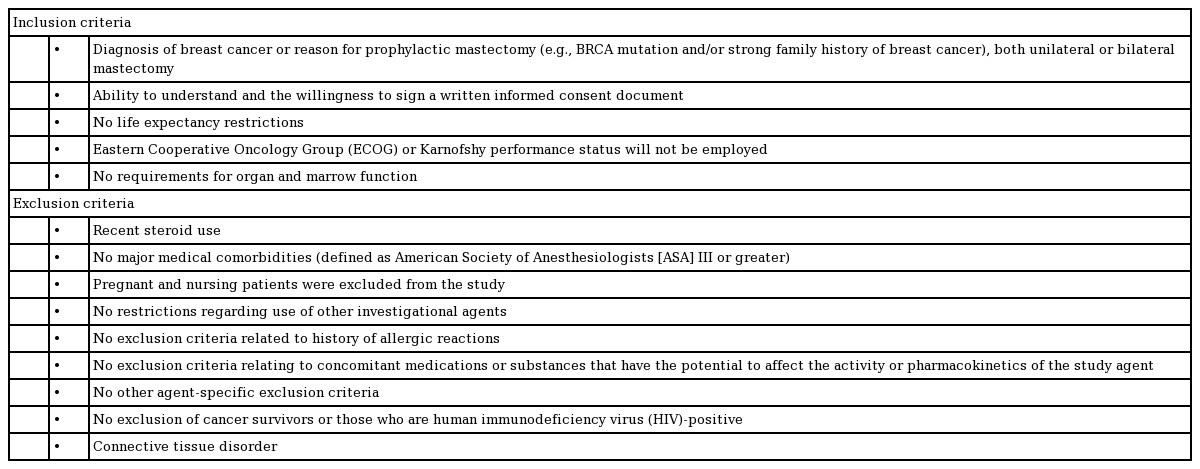
The Blossom Smart Expander trial was a prospective, single-center, single-surgeon study that assessed the efficacy and safety of the Marz Blossom Saline Delivery Assist Device. This study was conducted in compliance with the Institutional Review Board regulations and conformed to the Declaration of Helsinki (IRB-44367). Female volunteer patients meeting the inclusion criteria (Table 1), were enrolled from August 2018 to May 2020. Written informed consent was obtained from all patients. The data presented in this study are deidentified, but the data were not deidentified during the collection and analysis processes.
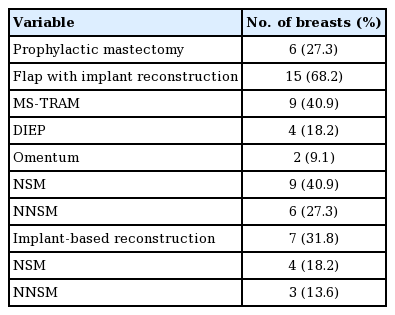
The participants of this study underwent either implant-based breast reconstruction or a combination of an autologous free flap with implant-based breast reconstruction following nipple-sparing mastectomy (NSM) or non-nipple-sparing mastectomy (NNSM). The free flaps used in the combination group included muscle-sparing transverse rectus abdominis myocutaneous (MS-TRAM) flaps, deep inferior epigastric perforator (DIEP) flaps, and greater omentum flaps (Table 2). Patients were seen by a clinician on a weekly basis during the expansion period to ensure their safety and to record pain scores using a numerical pain rating scale.
Operative technique
All breast reconstruction operations included in this study were performed by the senior author at a single center. The TE and Blossom system were inserted in the standard fashion. A Mentor Spectrum Adjustable Saline implant (Mentor, Santa Barbara, CA, USA) was used as the TE in all cases. The implant was selected based on the patient’s desired breast size and chest wall diameter.
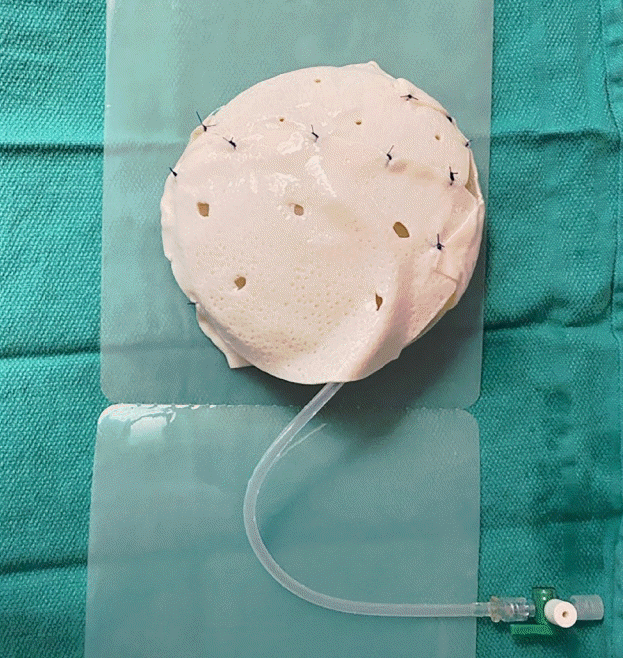
Implant coverage was achieved with either acellular dermal matrix (ADM), an MS-TRAM flap, a DIEP flap, or an omental free flap-ADM construct. For the implant-based cases, a sheet of ADM measuring 16×20 cm was fenestrated and contoured around the TE, which was inflated to approximately 90% of the final filling volume (Fig. 3) to ensure adequate room for future expansion. The TE was then deflated and the TE-ADM construct was placed into the subcutaneous pocket and secured to the pectoralis major fascia and inframammary fold. For the cases involving a combination of a flap (MS-TRAM, DIEP, or omental flap) with implant-based reconstruction, the TE-ADM construct was inserted into the prepectoral breast pocket, as described above, and the free flap was then draped over the TE. The choice of flap was determined based on tissue availability and the patient’s preference. The TE was inflated to 0%–30% of the final fill volume intraoperatively to minimize tension on the closure. The initial filling volume was determined intraoperatively according to capillary refill of the skin flaps. The TE external remote port fill tubing was exteriorized through a skin stab excision at the inferior lateral chest and secured to the skin with 3-0 nylon sutures. A number 15 Blake channel drain was placed in the subcutaneous pocket, brought out through a separate skin stab incision anterior to the TE fill tubing, and secured to the skin with 3-0 nylon sutures (Fig. 4). The wound edges were then reapproximated with interrupted 3-0 Vicryl sutures in the deep dermis and the epidermis was approximated with a running 4-0 Monocryl subcuticular stitch. All incisions were cleaned and dressed with the Dermabond Prineo skin closure system (Ethicon Inc., Somerville, NJ, USA).
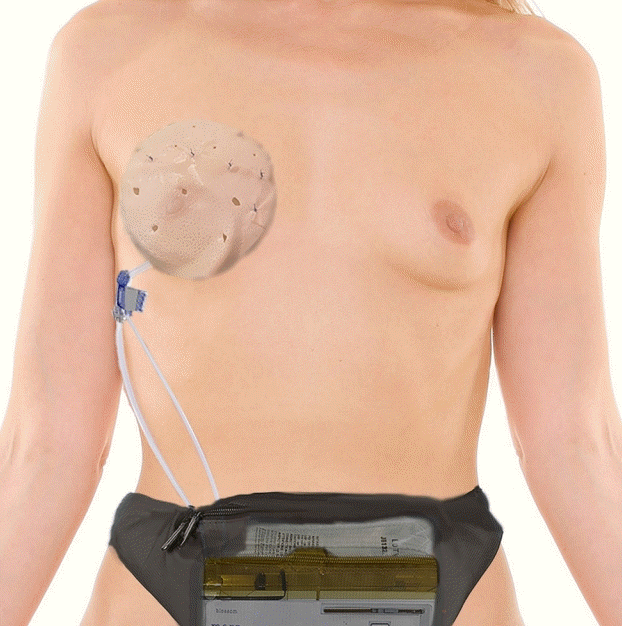
Implant coverage with ADM
A 16×20 cm sheet of acellular dermal matrix (ADM) is fenestrated and contoured around the tissue expander (TE), which is inflated to approximately 90% of the final fill volume. The ADM is secured around the TE using 2-0 polydioxanone sutures.
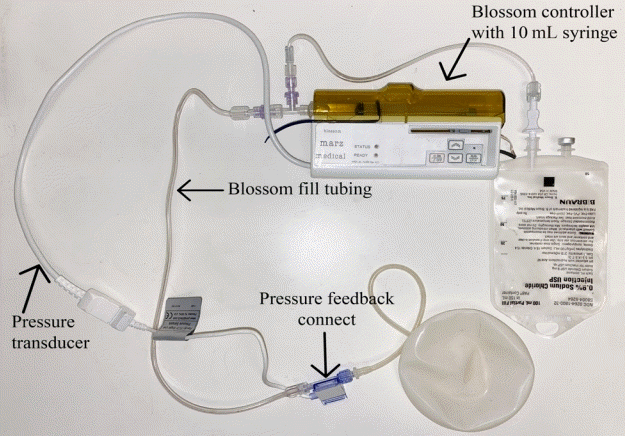
The Blossom system
A 250-mL saline bag and the Blossom controller are inserted into the designated pouch, which is secured around the patient’s waist as shown.
The TE remote port (by Mentor) was not used, and the TE fill tubing was connected to the sterile pressure feedback connector and fill tubing of the Blossom system. The Blossom fill tubing was capped and secured to the skin with Tegaderm (3M, Maplewood, MN, USA). The Prineo dressing was removed after 3 weeks. The drain was removed when the output was <30 mL over a 24-hour period for 2 consecutive days. All patients received perioperative intravenous antibiotics for 24 hours, followed by oral antibiotics until drain removal.
Tissue expansion
After allowing for adequate healing of incisions (2–3 weeks), patients were seen in clinic to initiate tissue expansion with the Blossom device. The remainder of the Blossom system was assembled and primed with sterile saline according to the manufacturer’s instructions. The Blossom controller was activated in the outpatient clinic. The fill rate was set at low (30 mL/day), medium (45 mL/day), or high (60 mL/day) depending on the degree of tissue laxity and perfusion of the breast envelope. The saline bag and Blossom controller were inserted into the designated pouch, which was secured around the patient’s waist as shown in Fig. 4. Once the TE (Mentor Spectrum saline implant) was inflated to the desired size, the Blossom controller was turned off and disconnected from the fill tubing. The TE fill tubing was then pulled caudally and perpendicularly to the skin until the entire tubing was removed through the existing skin opening. The TE self-sealed and could remain as a permanent saline implant.
Data collection and statistical analysis
The following outcome measures were recorded: time to full expansion, initial fill volume, volume of full expansion, device malfunction, incidence of complications, and patient-reported pain scores during expansion. The documented variables included patient age, medical comorbidities, and the use of chemotherapy and radiation therapy.
The time to full expansion was measured by calculating the number of days from the initiation of saline infusion with the Blossom device until complete delivery of the total programmed volume. Complications were categorized as major or minor. Major complications included infections requiring intravenous antibiotic therapy and any issue requiring hospitalization or surgical intervention (e.g., wound closure revision, surgical drainage of the expander pocket, and TE explantation). Minor complications included issues that did not require hospitalization or surgical intervention (e.g., hematoma, seroma, infections requiring oral antibiotics, delayed wound healing, and uncontrollable pain).
The primary endpoint of this study was successful tissue expansion to the desired volume. Breasts requiring TE removal prior to full expansion were considered failures. The distribution of data was quantified using standardized tables constructed in Excel (Microsoft Corp., Redmond, WA, USA). A descriptive statistical analysis including mean, standard error of the mean (SEM), median, range, and interquartile range (IQR) was also carried out in Excel. Inferential statistical analysis was performed to assess the significance of differences between participants who experienced complications and those who did not develop complications. We analyzed the study endpoints, outcome measures, and patient characteristics to assess correlations between variables and complication risk. The analysis was performed with GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA). The Fisher exact test was used to calculate two-tailed P-values. The paired t-test was used to assess patient-reported pain scores in the combination and implant groups. A P-value <0.05 was considered to indicate statistical significance.
RESULTS
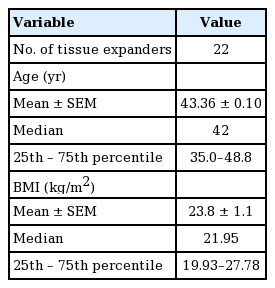
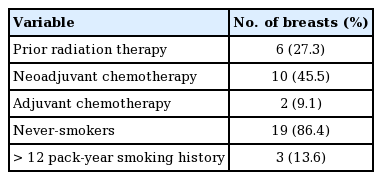
Fourteen patients (22 TEs; n=22), were included in this study. The participants’ age ranged from 30 to 66 years old (mean, 43.4±0.1) (Table 3). Fifteen of the TEs (68.18%) were inserted in combination with an autologous flap, and implant-based breast reconstruction was performed in the remaining seven cases (31.8%). Within the combination group (n=15), TE coverage was achieved with an MS-TRAM flap (n=9, 60%), a DIEP flap (n=4, 26.7%), or a greater omentum flap (n=2, 13.3%). Additionally, NSM and NNSM were performed in 60% (n=9) and 40% (n=6) of cases, respectively, within the combination group (Table 2). Within the implant only group (n=7), NSM and NNSM were performed in 57.1% (n=4) and 43.9% (n=3) of cases, respectively. Of the 22 TEs, 54.4% (n=12) were inserted into breasts treated with chemotherapy: 45.5% (n=10) into breasts treated with neoadjuvant chemotherapy, and 9.09% (n=2) into breasts treated with adjuvant chemotherapy (Table 4). A history of prior radiation was present in 27.3% (n=6) of the breasts.
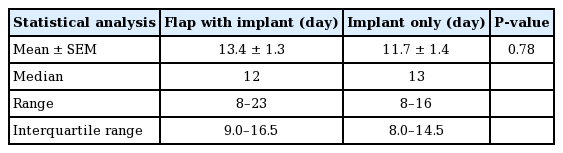
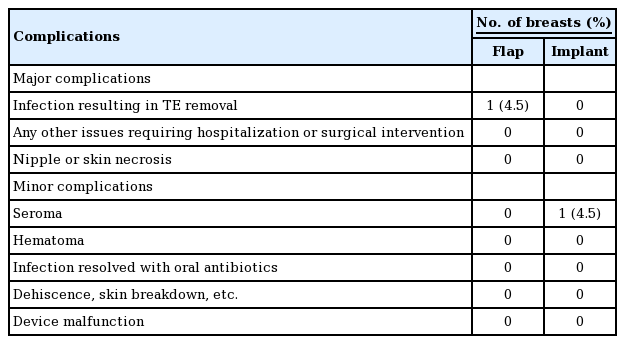
The average intraoperative fill volume of the TE was 16.7 mL (range, 0–100 mL). The mean time to full expansion was 13.4 days (SEM, 1.3 days) in the combination group and 11.7 days (SEM, 1.4 days) in the implant group (P=0.78) (Table 5). The overall complication rate of this study was 9.09% (n=2). There was one major complication (4.5%, n=1), requiring TE explantation (Table 6). In this case, the patient reinserted a drain that had been pulled out and subsequently developed cellulitis of the ipsilateral radiated breast. Her TE was explanted after a failed course of antibiotic therapy. A minor complication (4.5%, n=1), involved seroma formation following drain removal that resolved spontaneously.
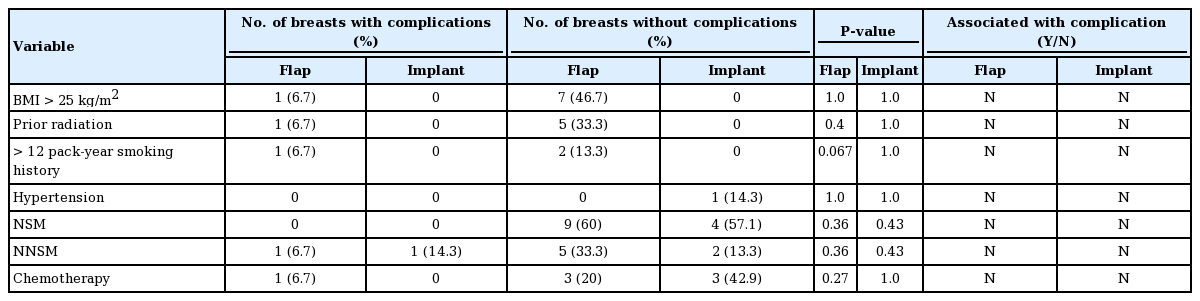
A body mass index (BMI) greater than 25 kg/m2, corresponding to the overweight and obese category, was not associated with the occurrence of complications in either group (Table 7). In the combination and implant only groups, current or past smoking history was not correlated with a higher complication risk (P=0.067 and P=1.0, respectively). A history of radiation therapy and chemotherapy was not correlated with a higher complication risk in either group (Table 7). The duration to full expansion, flow rate of the Blossom system, and initial and final fill volumes were not associated with higher complication rates.
The final fill volumes ranged from 100 to 450 mL (mean, 270±17.6 mL). The initial intraoperative fill volume, age, original breast cup size, and presence of hypertension did not affect the risk of complications in a statistically significant manner. There were no cases of device malfunction. In all cases, the fill tubing was detached from the TE after completion of expansion in the outpatient clinic without complications. One participant elected to keep the TEs as permanent saline implants. The remaining patients underwent exchange of TEs with silicone implants.
The maximum patient-reported pain scores during the expansion process were significantly higher in the combination group, (mean, 2.00±0.09) than in the implant group (mean, 0.29± 0.25; P=0.005) (Table 8). Preoperative and postoperative images from the combination flap and the implant-based breast reconstruction groups are shown in Figs. 5 and 6, respectively.
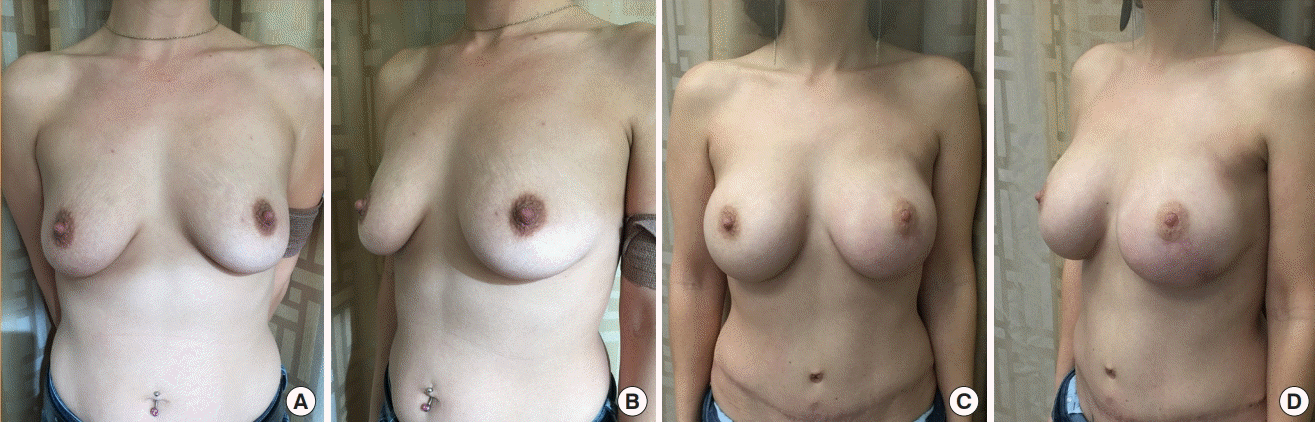
Combination flap with implant using the Blossom system
The patient was a 32-year-old woman with left breast cancer after neoadjuvant chemotherapy, left nipple-sparing mastectomy, postoperative radiation therapy, and delayed reconstruction with a left deep inferior epigastric perforator flap with prepectoral Mentor Spectrum saline implant and the Blossom system, along with contralateral right breast augmentation. The left implant final fill volume was 310 mL, and that of the right breast implant was 385 mL. (A, B) Preoperative anterior and lateral view. (C, D) Nine months after removal of the Blossom port and fill tubing following complete volume expansion with the Blossom system. The components were removed in the clinic without local anesthesia.

Two-stage implant reconstruction with the Blossom system
The patient was a 35-year-old woman with a history of CHEK2 mutation who underwent bilateral prophylactic nipple-sparing mastectomy and immediate breast reconstruction with prepectoral tissue expansion with the Blossom system. (A) Preoperative view. (B) Immediately after removal of the Blossom port and fill tubing following complete volume expansion with the Blossom system. (C) Six months following bilateral capsulotomy and implant (Natrelle Inspira smooth shell, cohesive silicone gel, extra full profile 420 mL) exchange along with fat grafting.
DISCUSSION
This study demonstrates the usability of an innovative device for expediting TE-implant based breast reconstruction. All of the study participants achieved full expansion in 23 days or less. In this pilot study, the mean time to full expansion was 13.4 days and 11.7 days in the combination and implant groups, respectively. In contrast, the reported average duration for conventional subcutaneous TE expansion is 79.4 days [7]. The Blossom system facilitates an at-home tissue expansion process with the potential to decrease patients’ burdens in terms of transportation barriers, as well as the pain and extended duration of time associated with conventional tissue expansion.
Minimizing the total duration to achieve complete breast reconstruction will profoundly impact patients’ quality of life. The significant improvements in psychosocial, sexual, and physical well-being between patients who undergo mastectomy and breast reconstruction compared to those who undergo mastectomy alone have been well established [8]. Furthermore, these findings were echoed by Al-Ghazal et al. [9], who reported that patients who underwent immediate breast reconstruction were less distressed and had better psychosocial well-being than those who opted for delayed reconstruction.
Conventional tissue expansion for two-stage breast reconstruction carries a decades-long history of demonstrated safety with favorable aesthetic outcomes. However, it confers several disadvantages, including the pain and anxiety associated with serial injections, depletion of health care resources, interference with patients’ work or daily activities, and an increased risk of TE leakage/rupture [10]. In contrast, the Blossom system obviates the need for multiple clinic visits, painful injections, and abrupt large volume expansions. The innovative technology allows for pressure-regulated expansion of the TE in a safe, slow, and continuous manner and prevents undue stress to the overlying tissue. There were no observed cases of nipple or skin flap necrosis in this study.
There has been an increased demand at our institution for procedures combining a flap with implant-based breast reconstruction. Patients with relatively low BMI lack the subcutaneous volume to provide adequately-sized free flaps for breast reconstruction. Furthermore, it is not uncommon for patients to request augmented breast volumes with reconstruction. Draping autologous tissue over the implant in combination flap reconstructions creates a natural and aesthetically favorable outcome, and the Blossom system allows (unfilled) TE insertion in conjunction with flap anastomoses, minimizing tension on the vascular pedicle. The small yet continuous infusion of saline through the Blossom system permits skin and nipple perfusion to the thin mastectomy skin flaps. Additionally, the Blossom system precludes the need for percutaneous injections and subsequent flap puncture, which may lead to vascular pedicle injuries.
This study was limited by a small sample size (n=22), and its single-center, single-surgeon design. It was a pilot study aimed to demonstrate the efficacy and safety of the device. The limitations of the Blossom system include patients’ discomfort with continuously wearing the device, particularly the external pouch housing the saline bag. Therefore, this system may not be ideal for all patients.
As presented in this study, the Blossom system may accommodate single-stage breast reconstruction procedures. A study by Azzi et al. [11] demonstrated successful single-stage breast reconstruction utilizing Mentor Spectrum adjustable implants. However, Azzi et al. did not exteriorize the TE port to accommodate fill tubing removal in a clinical setting, as was done in our study. The topic of external ports with tissue expansion is controversial. External ports require routine care, and appropriate patient selection is important. Studies have demonstrated comparable and even superior outcomes in comparison to internal ports [12,13]. The overall complication rate of this study, including minor complications, was 9.09%. The reported complication rates of two-stage breast reconstruction with conventional saline TEs range from 20% to 45% [12-21]. This suggests that the Blossom system has a comparatively low overall complication risk. The Blossom system does not carry the same repeated risk of microbial inoculation that is present with serial port injections. On average, the fill tubing is removed within 2 weeks; therefore, it does not impose more risk than standard surgical drains.
Additional large-scale studies are required to corroborate the low complication rates in this study and to establish clear indications for the Blossom system. However, since the Blossom system is a substantially equivalent, yet improved version, of its predecessor (the McGhan Tissue Expander Fill Kit), which was granted 510k (K853014) [22] clearance by the Food and Drug Administration in 1985, the Blossom system has been deemed safe and exempt from requiring premarket approval. This is not an investigational medical device and is commercially available.
Tseng et al. [23] found that patients with breast cancer residing in rural areas were more likely to undergo mastectomy, but less likely to undergo breast reconstruction than their urban counterparts. These disparities in breast reconstruction were also demonstrated by DeCoster et al. [24], who reported that patients from rural areas were less likely to undergo breast reconstruction than their urban counterparts (31.1% and 13.4%, respectively). Disparities in undergoing breast reconstruction are multifactorial, and negative predictors include greater than a 32-km distance to a reconstructive surgeon, lack of an appropriate preoperative consultation, non-possession of private insurance, and non-White race [23-25]. We believe that the Blossom system may be a practical option for patients residing in rural areas and hope that it will help to decrease disparities.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Stanford University Medical Center (IRB No. IRB-44367) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: DH Nguyen. Data curation: DG Rochlin. Formal analysis: YK Choi. Methodology: DH Rochlin. Project administration: DH Rochlin. Visualization: YK Choi. Writing - original draft: YK Choi. Writing - review & editing: YK Choi, DH Rochlin, DH Nguyen.