The use of negative-pressure wound therapy over a cultured epithelial autograft for full-thickness wounds secondary to purpura fulminans in an infant
Article information
Abstract
Purpura fulminans is a serious condition that can result in severe morbidity in the pediatric population. Although autologous skin grafts remain the gold standard for the coverage of partial- to full-thickness wounds, they have several limitations in pediatric patients, including the lack of planar donor sites, the risk of hemodynamic instability, and the limited graft thickness. In Singapore, an in-house skin culture laboratory has been available since 2005 for the use of cultured epithelial autografts (CEAs), especially in burn wounds. However, due to the fragility of CEAs, negative-pressure wound therapy (NPWT) dressings have been rarely used with CEAs. With several modifications, we report a successful case of NPWT applied over a CEA in an infant who sustained 30% total body surface area full-thickness wounds over the anterior abdomen, flank, and upper thigh secondary to purpura fulminans. We also describe the advantages of using NPWT dressing over a CEA, particularly in pediatric patients.
INTRODUCTION
Purpura fulminans is a serious condition that can result in severe morbidity in the pediatric population [1]. Due to the particular vulnerability of the pediatric population, resurfacing extensive wounds in these patients poses a unique challenge to the reconstructive surgeon [1]. Although autologous skin grafts remain the gold standard for the coverage of partial- to full-thickness wounds, they have several limitations in pediatric patients, including the lack of planar donor sites, the risk of hemodynamic instability, and limited graft thickness. To overcome the insufficiency of donor tissue, a plethora of skin substitutes are available, including xenografts, synthetic bilayers, acellular allografts, composite allografts, and cultured epithelial autografts (CEAs) [2]. Each of these skin alternatives has its advantages and disadvantages, and the final choice depends on physicians’ experience and institutional practices. In Singapore, an in-house skin culture laboratory for CEA culture has been available since 2005 [3]. The process for producing a CEA was adopted from the methods pioneered by Rheinwald and Green [4]. It involves growing keratinocytes from a small skin biopsy (1–4 cm2) to obtain a large amount of epithelium sheets within a 3 to 4 weeks period. The cultured cells grow on a fibrin matrix that provides support and enables straightforward delivery of the unicellular sheets onto the wound bed. Once placed, a CEA needs to be kept completely immobile and on non-pressure sites to prevent graft loss [5]. However, the widespread use of CEAs has been hindered by their fragility, variable take rates, and high cost [6].
We report a successful case of negative-pressure wound therapy (NPWT) applied over a CEA in an infant who sustained 30% total body surface area full-thickness wounds over the anterior abdomen, flank, and upper thigh secondary to purpura fulminans. The effectiveness of both NPWT and CEAs for pediatric wounds have been separately documented in the literature. However, the fragile nature of CEAs is a deterrent for the combined use of these two modalities. In addition to assisting with stimulation of healthy granulation tissue, the major advantage of vacuum-assisted closure (VAC) therapy, particularly in the pediatric population, is exudate control and the ease of nursing. Based on the recommendation of the Singapore Bioethics Advisory Committee guidelines for institutional review boards on research involving human subjects (November 2004), this study was exempted from review by the Centralized Institutional Review Board of SingHealth, Singapore. Written consent was obtained from the patient’s legal guardian.
CASE
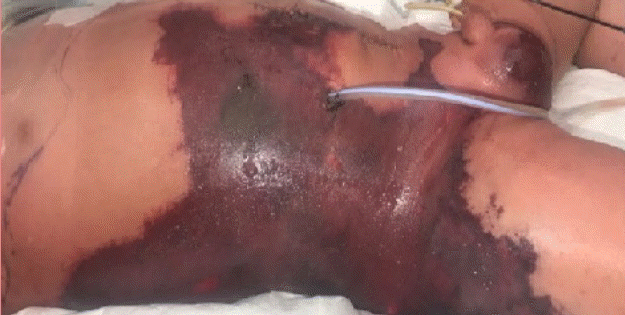
Purpura fulminans developed in a 1-year-old infant secondary to enterocolitis sepsis after an uneventful laparoscopic endorectal pull-through procedure for Hirschsprung disease. Septic shock secondary to enterocolitis developed on the third postoperative day, requiring inotropic support and mechanical ventilation. Subsequently, an exploratory laparotomy was performed and a colostomy was created to decompress his digestive system. After laparotomy, he developed a purpuric lesion over the right anterior abdomen, flank, and lateral thigh (Fig. 1) affecting 30% of his total body surface area. The histological diagnosis of purpura fulminans was confirmed based on a skin biopsy 5 days post-laparotomy. The affected areas were excised serially and debrided multiple times over a period of 3 weeks, leaving an extensive full-thickness wound exposing muscle and deep fascia (Fig. 2). On the second debridement (first week), a 2 × 2 cm skin biopsy was harvested for CEA in preparation for wound coverage. The wound was covered with a VAC dressing after every debridement for wound bed preparation, exudate management, and ease of nursing care. After a total of six serial debridements, the wound was deemed clean and ready for grafting (Fig. 2). At this time, four 10 × 10 cm sheets of CEA showed confluence, indicating readiness for application [5]. The CEA was applied together with a 6/1,000-inch autologous split-thickness skin graft (SSG) harvested from the right medial thigh and meshed to a 1:6 ratio of expansion as per the modified JACE technique described by Matsumura et al. (Fig. 3) [7,8]. The flanks and back were at higher risk of shearing and cell loss than the anterior abdomen, which is why we decided to limit the use of this technique to the anterior abdomen [9]. Consequently, Integra (Ethicon; Johnson & Johnson Medical, Norderstedt, Germany) was used as a resurfacing option for the flank and back. Integra is an artificial dermal substitute, which is a bilayer membrane system consisting of a superficial layer (temporary silicone layer) and a deep layer (bovine collagen and glycosaminoglycan matrix). The superficial layer is removed and replaced by an SSG after integration of the deep dermal layer.
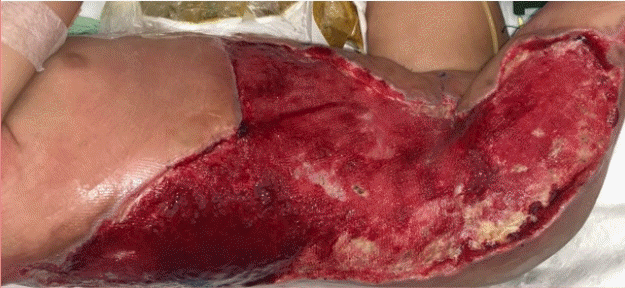
Photograph of purpuric lesions
A homogeneous purpuric lesion affected 30% of the total body surface area.
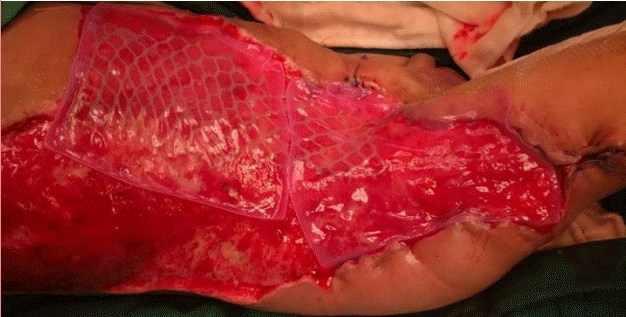
Application of CEA with widely meshed SSG
Application of a cultured epithelial autograft (CEA) with a widely meshed split-thickness skin graft (SSG) at a 1:9 ratio after 3 weeks of debridement.
Upon application of Integra and CEA as described above, NPWT was applied with the VAC system (KCI Inc., St. Paul, MN, USA) for three reasons: (1) to enable straightforward exudate management, (2) to secure the grafts and Integra firmly over a highly mobile wound bed, and (3) to protect the fragile unicellular layer of keratinocytes. However, applying negativepressure dressings over newly grafted skin and keratinocytes is likely to peel off the fragile layer owing to mechanical pulling suction, especially during removal. This is analogous to peeling a highly adherent plaster off highly fragile skin. To address this concern, the authors modified the negative-pressure dressing in two ways: (1) by replacing the Granufoam VAC with Mepilex dressing (Mölnlycke Inc., Gothenburg, Sweden) over areas with CEA and skin grafts, and (2) lowering the negative-pressure suction from 125 mmHg to 50 mmHg while using a low-intensity setting.
Granufoam is a hydrophobic polyurethane sponge with 400- micron spaces that was designed to facilitate granulation tissue ingrowth. However, the authors were concerned that using this foam over the thin and fragile sheets of keratinocytes would lead to more shearing, affecting the graft take. In contrast, Mepilex consists of a hydrophilic soft silicone sponge with much smaller spaces with an embedded non-adherent, but porous silicone layer known as the Safetac technology. Mepilex foam dressings are commonly used for skin graft donor sites and for the protection of fragile skin.
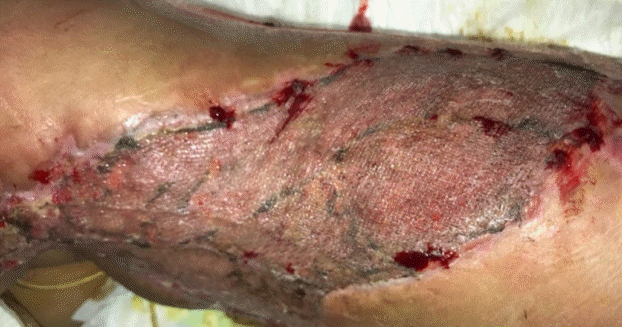
Post-CEA application day 7
Wound inspection was carried out 7 days after grafting. The CEA/SSG was successfully grafted (almost 100%) (Fig. 4). Integra was adherent to the wound bed, but the silicone layer remained inseparable. New Mepilex foam dressings were applied over the CEA/SSG areas, while KCI VAC Granufoam was applied again over the Integra-covered areas.
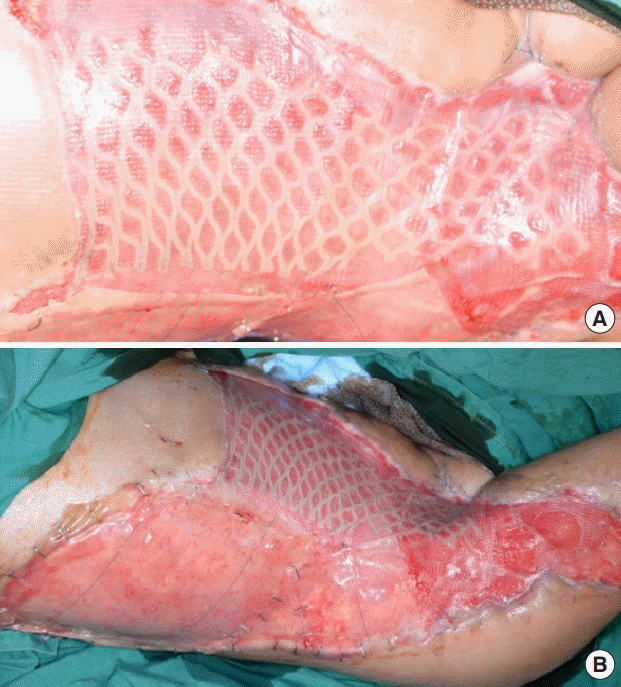
Anterior abdomen wound grafted with a CEA/SSG
(A) Complete take 1 week after application. (B) The fully-taken cultured epithelial autograft with a split-thickness skin graft (CEA/ SSG), with Integra adherent to the wound.
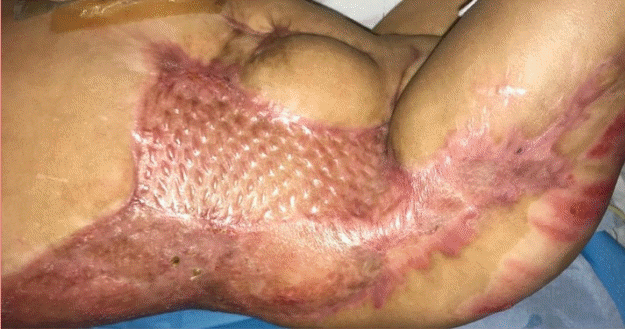
The same wound inspection process was carried out weekly. At week 3, the wound areas with Integra were ready for grafting. During this time, the SSG was harvested from the back and posterior thigh at a thickness of 9/1,000 inch and meshed to 1:1.5 ratio. The skin graft was secured with staples over the right back, flank, and upper thigh areas. The entire wound was then covered with a negative-pressure dressing at the same setting.
At week 4, the Integra with the SSG that was applied 7 days prior showed complete graft take (Fig. 5). Meanwhile, the anterior abdomen region, where the CEA was applied with the SSG 4 weeks prior, had healed and the intervening spaces between the meshed skin with the CEA showed stable re-epithelialization. NPWT was halted at week 4 and the wounds were dressed with normal protective dressing. Fifty-two days after application of the CEA, the patient was discharged with all wounds fully healed.
One-year postoperative follow up
Scarring of both the donor site and wound is a major consideration in patients with full-thickness wound defects. In this patient, the wound extended along the flanks to the thigh over the right hip joint. The patient developed hypertrophic scarring over the skin graft donor sites and the right thigh at the junction between the CEA and Integra application (Fig. 6). Interestingly, the anterior abdomen where CEA was applied with the meshed skin graft healed with minimal hypertrophic scarring (Figs. 6, 7). One year after resurfacing and conservative scar management, on the anterior abdomen where the CEA was applied with the extended mesh graft, the scar was soft and had a Vancouver Scar Scale score of 5 (Fig. 7). The scar outcome for the back, where Integra and the SSG were applied, showed a similar result, with a Vancouver Scar Scale score of 5 (Fig. 7). This is consistent with previous reports that have concluded that CEA with an expanded mesh skin graft yields scarring outcomes comparable to those of conventional skin grafting [10]. This also suggests that the scarring was comparable to the use of a conventional meshed skin graft with Integra, as indicated by Vancouver Scar Scale scores (Fig. 7). The hypertrophic scarring that developed at the junction between the CEA and Integra application improved with scar management techniques such as pressure garments and scar massage.
DISCUSSION
Purpura fulminans is a rare, rapidly progressive, and potentially fatal thrombotic disorder that typically affects neonates and children. Purpura fulminans may occur as a result of an acute episode of severe sepsis, following an acute systemic response for which there is systemic activation of the coagulation and complement pathways, as well as endothelial dysfunction. This pathological state is commonly known as disseminated intravascular coagulation, which is followed by microvascular thrombosis in the dermis and hemorrhagic infarction of the skin [1]. In this patient, the mainstay of treatment was full supportive care in the intensive care unit, treatment of underlying sepsis with broad-spectrum antibiotics, and early surgical excision of the necrotic skin.
Infants around 1 year of age are a highly vulnerable group with a much smaller volume-to-surface area ratio than adults. In addition to the problem of scarring, extensive skin graft harvest for coverage of large surfaces can lead to catastrophic loss of fluid, electrolytes, protein, and body heat with an increased risk of mortality, morbidity, and prolonged hospitalization [11,12]. Furthermore, the skin of neonates, infants, and children possesses a relatively thin dermal layer, minimizing the depth of tissue that can be harvested [12].
The identification of an appropriate donor site is a critical consideration in deciding to perform an autograft [12]. The donor site should ideally be a relatively planar area of healthy skin that can be hidden by clothing to reduce the cosmetic impact of potential scarring resulting from surgical harvest of the autograft. In pediatric patients, this can be challenging due to their relatively small total body surface area [12].
In this patient, there were several important considerations for resurfacing such an extensive percentage of the body surface area. First, since the infant was already critically ill and hemodynamically unstable, the resurfacing procedure had to be performed quickly to reduce the risk of morbidity. As the wound was already extensive, the use of an SSG for immediate resurfacing may have exacerbated the hemodynamic instability, as a donor site corresponding to 30% of total body surface area would have increased the wound surface area to 60%. The next consideration was the depth of the wound. Since the wounds were fullthickness over the flank and right hip, exposing deep fascia and muscles, avoiding scar contracture was an important consideration; therefore, the decision was made to use Integra as a dermal substitute in those areas to reduce the risk of scar contracture and to improve skin mobility in that region [13,14]. The decision to use CEA enabled us to resurface the large intervening areas of the maximally expanded autologous skin, significantly reducing the extent of the SSG required for resurfacing [5]. The CEA was used only for the anterior abdomen, instead of the flanks and back, where the risk of shearing is higher.
NPWT has been widely used with SSGs and has been shown to improve graft take [15]. However, to date, negative-pressure therapy has not been used with CEA. A review of the peer-reviewed English-language literature in PubMed using the keywords “cultured epithelial autografts” and “negative pressure” performed by the authors confirmed that no reports had been published on the use of this combination for wound management.
The authors postulate that it is logical for most surgeons to avoid the use of NPWT in combination with CEAs due to the fragility of unicellular autologous keratinocytes. In the extremities, the use of negative-pressure therapy ensures the immobilization of skin grafts, thereby improving graft take. In this patient, given the age, location and extent of the wound, we were unable to find effective methods other than negative-pressure therapy to immobilize the infant’s skin grafts over the trunk while simultaneously managing the exudates effectively. Wounds with a large surface area also necessitate daily changes of dressing owing to the high-volume egress of exudates, which may inadvertently place a burden on the nursing team. Furthermore, the VAC dressing ensured accurate estimation of fluid loss and reduced fluid loss through evaporation, thereby enabling more efficient fluid management, which is another important component of the management of pediatric patients. Sealing the wound also helped to reduce the risk of contamination, particularly in this patient, who also had a colostomy adjacent to the wound.
To minimize the risk of damage to the CEA, the CEA sheets on the SSG were protected with Mepilex foam dressing, which has a smaller pore size than the VAC sponge, but is equally effective in enabling a seal for the VAC. VAC therapy was also set at a very low pressure (50 mmHg). We therefore can report that VAC therapy was successfully used without damage to a CEA; therefore, this technique can be considered as an option for dressing extensive wounds in the pediatric population to ease nursing care.
Herein, we report the first successful clinical use of a CEA with modified NPWT. The use of CEA with widely-meshed SSG showed good scar outcomes. The authors propose that more clinical experience with the use of NPWT on CEAs would provide further evidence on the efficacy and safety of this technique.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient’s legal guardian provided written informed consent for the publication and the use of his images.
Author contribution
Conceptualization: AWC Chua, KY Chew, GCW Kang, S Ramachandran. Methodology: BK Tan. Project administration: LW Chiang, S Ramachandran. Writing - original draft: BKL Goh. Writing - review & editing: BKL Goh.