Comparison of breast volume change between oncoplastic breast-conserving surgery with radiation therapy and a simultaneous contralateral balancing procedure through the inverted-T scar technique
Article information
Abstract
Background
Reduction mammoplasty or mastopexy is performed as an additional balancing procedure in patients with large or ptotic breasts who undergo breast-conserving surgery (BCS). Radiation therapy on breasts that have undergone surgery may result in changes in the volume. This study presents a comparative analysis of patients who received post-BCS balancing procedures to determine whether volume changes were larger in breasts that received radiation therapy than on the contralateral side.
Methods
Thirty-six participants were selected among patients who received BCS using the inverted-T scar technique between September 2012 and July 2017, were followed up for 2 or more years, and had pre-radiation therapy computed tomography images and post-radiation therapy images taken between 12 and 18 months after completion. The average age of the participants was 53.5 years, their average body mass index was 26.62 kg/m2.
Results
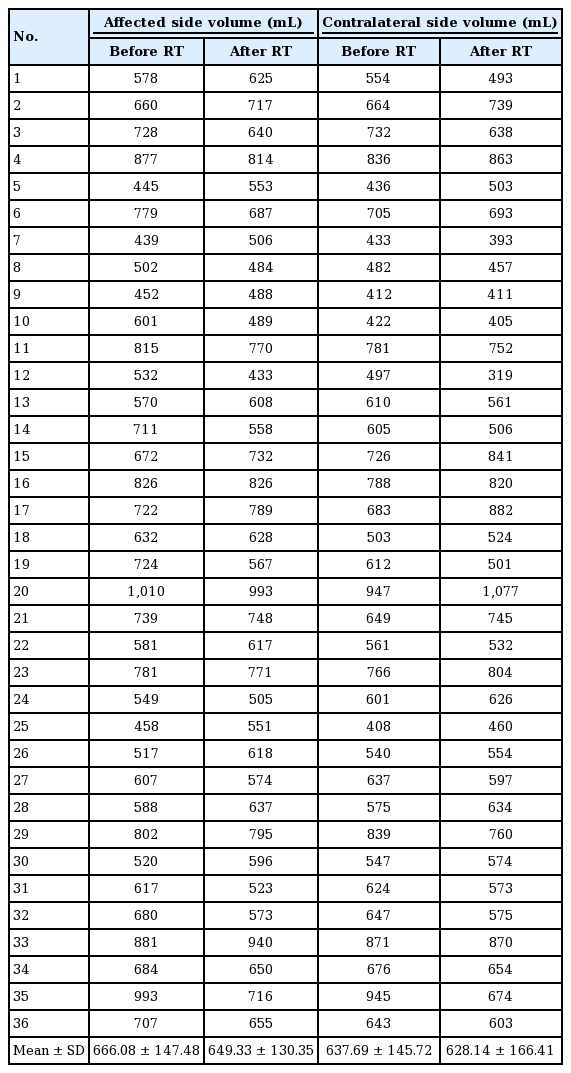
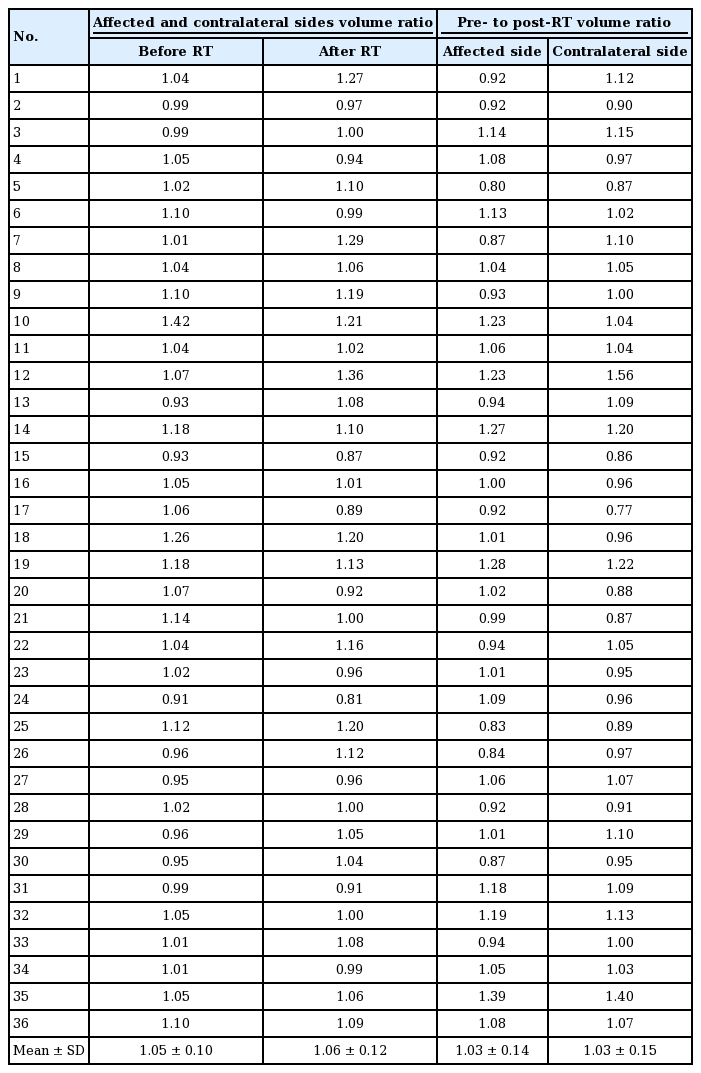
The pre- and post-radiation therapy volumes of the breasts receiving BCS were 666.08±147.48 mL and 649.33±130.35 mL, respectively. In the contralateral breasts, the volume before radiation therapy was 637.69±145.72 mL, which decreased to 628.14±166.41 mL after therapy. The volume ratio of the affected to the contralateral breasts was 1.05±0.10 before radiation therapy and 1.06±0.12 after radiation therapy.
Conclusions
The ratio of the volume between the two breasts immediately after surgery and at roughly 18 months postoperatively was not significantly different (P=0.98). For these reasons, we recommend a simultaneous single-stage balancing procedure as a reasonable option for patients who require radiation therapy after BCS without concerns regarding volume change.
INTRODUCTION
The number of patients receiving breast-conserving surgery (BCS) is on the rise, as BCS plays a crucial role in aesthetic breast reconstruction and the early detection of breast cancer is becoming more common [1-3]. The inverted-T scar technique, a type of BCS, is used for ptotic or large breasts in procedures involving reconstruction of the abnormal breast and simultaneous mastopexy or reduction mammoplasty on the contralateral side as a balancing procedure to obtain aesthetically symmetric results.
The contralateral balancing procedure can be either done simultaneously with the reconstruction procedure or as a secondary procedure after reconstruction [1,2,4]. Carrying out the balancing procedure simultaneously with the reconstruction procedure may generally increase the risk of asymmetric breasts compared to performing it as a delayed secondary procedure. However, previous research, including a study conducted by Smith et al. [1], reported good aesthetic results without additional procedures when balancing was performed immediately.
BCS requires adjuvant radiation therapy, which can cause changes in breast volume. In order to achieve symmetry between two breasts with an immediate balancing procedure, the normal breast should be reconstructed to be slightly smaller considering the potential for volume contraction after radiation therapy [5]. Very few studies have reported volume changes using objective measures [5]. Against this background, this study aimed to provide a reference for determining the extent of excision on the normal-side breast by comparing volume changes in breasts that received radiation therapy with those in the contralateral breasts among patients who received an immediate balancing procedure using the inverted-T scar technique.
METHODS
Participants
In total, 48 patients received BCS using the inverted-T scar technique for the abnormal breast accompanied by a simultaneous balancing procedure using the same method between September 2012 and July 2017. Among them, 36 participants were selected, with the exclusion of patients who received an extended latissimus dorsi flap, implant, or acellular dermal matrix or patients who did not undergo a follow-up computed tomography (CT) scan (Siemens, Munich, Germany) within 2 years postoperatively. The average age of the patients was 53.5 years, and their average body mass index was 26.62 kg/m2. The purpose of the balancing procedure was reduction mammoplasty in 35 cases and mastopexy in the remaining case. The excised weight for reduction was between 35 g and 675 g, except for the mastopexy case, and the excision volume between the two sides differed by 0 to 200 g. In 12 patients, the difference was 50 g or higher due to asymmetry between both sides. All patients received adjuvant radiation therapy and 30 patients underwent chemotherapy prior to radiation therapy. Only patients who had CT scans taken both before radiation therapy and 12 to 18 months after completing radiation therapy were selected as participants. Since breast volume is susceptible to changes after the completion of radiation therapy in response to changes in eating habits, exercise, and hormone therapy, the effects of radiation therapy were confirmed by comparing the volume of both breasts before and after radiation therapy. The reconstruction procedures were performed by one surgeon (SBN) and the CT measurements of breast were confirmed by single radiologist (KSC).
Radiation therapy
Radiation therapy was performed after surgery and the completion of systemic cancer treatment. Radiation therapy was administered to whole breast with or without regional lymphatics, using a 4–15 MV X-ray and/or 6–16 MeV electron beam from a linear accelerator (Varian Medical Systems, Palo Alto, CA, USA). Total radiation doses of 4,500–5,080 cGy in fractions of 180–250 cGy were typically administered, and boost doses of 540–2,540 cGy were directed to the tumor bed.
Surgical method
Prior to surgery, the design was made with the patient in a sitting position using the inverted-T scar technique, and the final excision area was marked. Surgery was performed under general anesthesia. The breast surgeon performed partial mastectomy after excision along part of the design, and then performed a reconstruction using the remaining breast tissue and a deepithelialized cutaneous flap to minimize uneven results. The non-lesion side was restored with a focus on maintaining symmetry using the inverted-T scar technique and excising the same area as the other side, with a similar excision volume. For breasts that were already asymmetric before surgery, the initial correction was made at the preoperative design stage, followed by a secondary correction during surgery with the patient in the sitting position by controlling the excision volume so that both sides were as close in size as possible. The weight of the removed tissue was measured with a digital scale.
Breast volume measurements
Breast volume was measured using CT images (Fig. 1). Patients were instructed to take a supine position with arms abducted and a lead wire was attached on the body surface to doublecheck the boundaries of the breasts by appearance. No contrast medium was used, and images were taken at a 5-mm slice thickness including the entire chest area. Special software for radiation image analysis (MIM Maestro ver. 6.6; MIM Software Inc., Cleveland, OH, USA) was used for image registration to compare the images taken before and after surgery. All measurements were performed by a single radiation oncologist to minimize individual differences in measurements. On each CT set, breasts were delineated according to ESTRO (European Society of Therapeutic Radiology and Oncology) guideline and Danish guideline, with modification of cranial and caudal definition to maintain objectivity. Following anatomical borders were used: (1) cranial, 5 cm above nipple; (2) caudal, 5 cm below nipple; (3) ventral, skin surface; (4) dorsal, anterior surface of major pectoral muscle or costae and intercostal muscles where no muscle exist; (5) medial, lateral to the edge of sternum; or (6) lateral, anterior to the lateral thoracic artery.
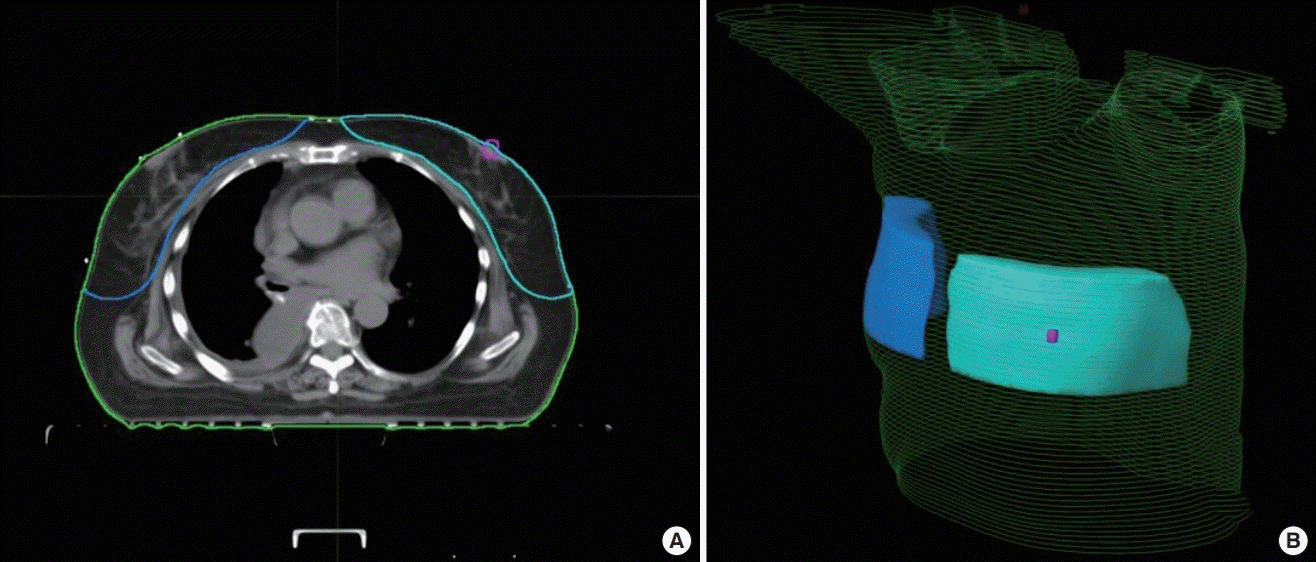
Breast volume measurements using CT images
Example of breast volume measurements on simulation computed tomography (CT) of patient who underwent right breast-conserving surgery. A transverse slice is shown (A), with corresponding projection view (B). Blue, ipsilateral breast; cyan, contralateral breast; magenta, contralateral nipple.
Statistical analysis
The ratio between the volume of the breast on which BCS was performed and that of the contralateral breast was calculated from the pre-radiation therapy CT scan, and the same ratio was calculated from the CT scan taken between 12 and 18 months post-radiation therapy. The Shapiro-Wilk test was conducted to test whether these values had a normal distribution, and the preradiation therapy values (P=0.07) and the post-therapy values (P=0.69) yielded P-values larger than 0.05. Therefore, both parametric (paired t-test) and non-parametric (Wilcoxon signedrank test and the sign test) methods were used. SPSS version 21.0 (IBM Corp., Armonk, NY, USA) was used.
RESULTS
The pre- and post-radiation therapy volumes of the breasts receiving BCS were 666.08±147.48 mL and 649.33±130.35 mL, respectively. In the contralateral breasts, the volume before radiation therapy was 637.69±145.72 mL, which decreased to 628.14±166.41 mL after therapy. When the breast affected by cancer was compared to the contralateral breast pre- and postradiation therapy, it was observed that the volumes of both sides decreased on CT imaging after radiation therapy, showing statistically significant changes (Pipsi=0.004, Pcont=0.002) (Table 1). However, no statistically significant change was found in the volume ratio of the affected to the contralateral breasts, which was 1.05±0.10 before radiation therapy and 1.06±0.12 after radiation therapy (Table 2). To verify these results statistically, the paired t-test (35 degrees of freedom, P=0.97), Wilcoxon signedrank test (P=0.81), and sign test (P=0.61) were performed, and all the results indicated equivalence. The pre- to post-radiation therapy ratio was 1.03±0.14 on the affected side, and 1.03±0.15 on the contralateral side.
In the clinical results from patients with at least 2 years of follow-up, both patients and surgeons were satisfied with the breast shape in 33 cases (Figs. 2, 3). A slight asymmetry in breast shape resulting from a deformity following radiation therapy was observed in one case. Three patients with a contraction deformity presented with a thin residual skin flap immediately after mastectomy, but these three patients were not dissatisfied with the shape of the breast and did not request reoperation. In addition, one patient wanted correction of a dog ear, one patient requested scar correction, and one patient wanted correction of nippleareolar complex deviation. The post-radiation therapy volume was larger than that of the contralateral side in one case.
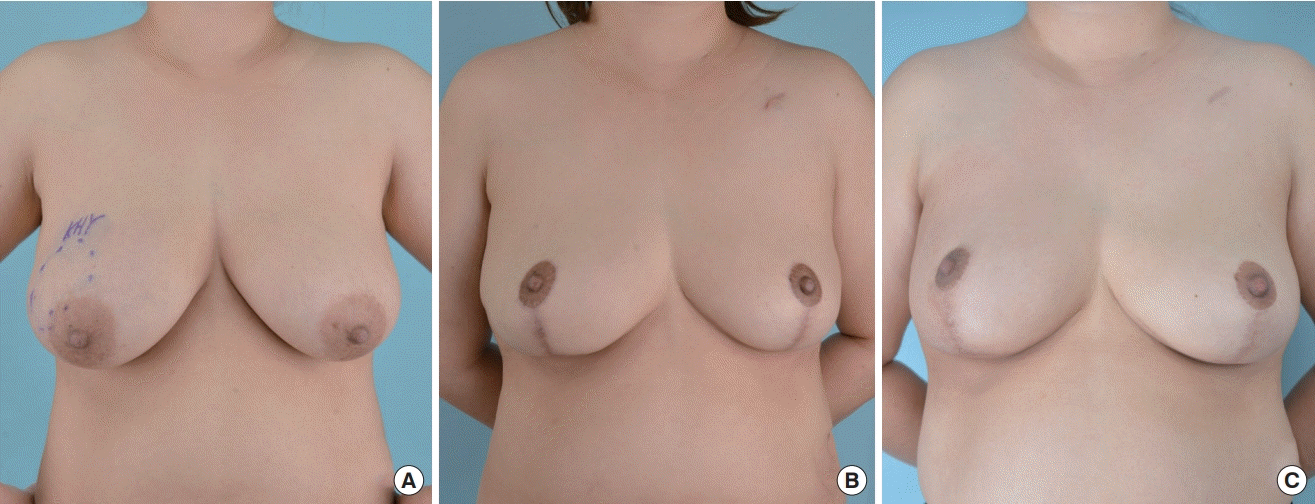
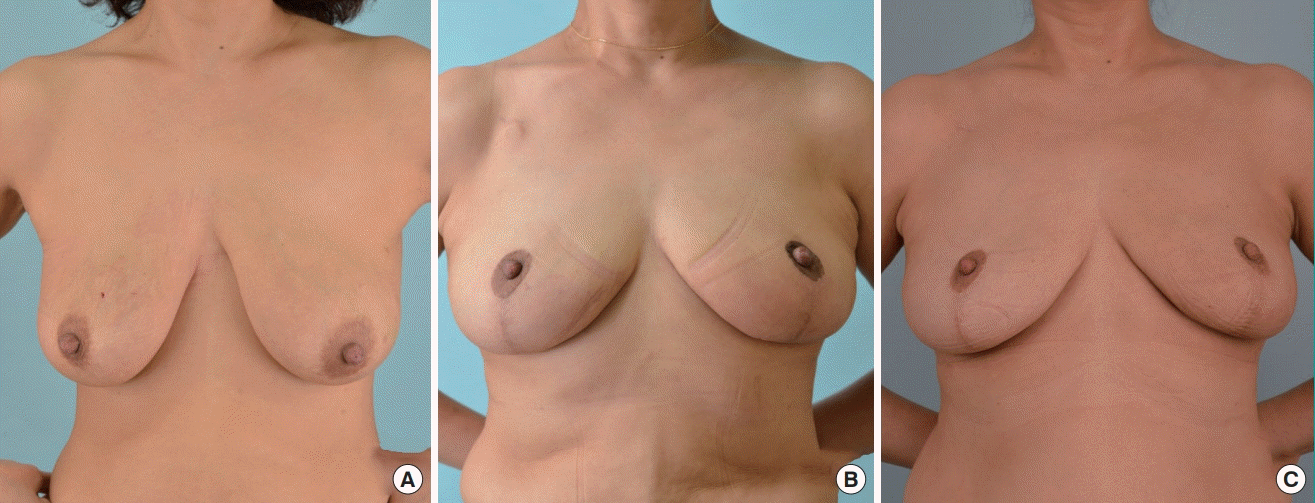
Follow-up photographs after reconstruction
The patient was 34 years old and had right breast cancer (T2N0M0). Her body mass index was 27.7 kg/m2. The excised volume was 242 g on right side and 248 g on left side. Photographs were taken at (A) pre-surgery, (B) pre-radiotherapy, and (C) 3-year post-radiotherapy.
Follow-up photographs after reconstruction
The patient was 51 years old and had left breast cancer (T2N0M0). Her body mass index was 20.4 kg/m2. The excised volume was 119 g on right side and 172 g on left side. Photographs were taken at (A) pre-surgery, (B) pre-radiotherapy, and (C) 3-year post-radiotherapy.
DISCUSSION
An important goal of breast reconstruction surgery is to achieve symmetry. However, since each individual has a unique breast shape, when performing reconstruction surgery, it is challenging to reconstruct a symmetric shape resulting in patient satisfaction. As it is thought to be a safe way of achieving symmetry, many surgeons still prefer to perform a balancing procedure on the contralateral side as a second-stage operation [2,4,6]. However, secondary revision breast surgery imposes an economic and psychological burden on the patient. In addition, it has been reported that the outcomes did not differ between balancing procedures performed simultaneously with the first breast reconstruction and those performed as a staged operation in terms of minor surgical complications or major complications such as transfusion, deep vein thrombosis, and readmission.
The author performed oncoplastic surgery with the inverted-T scar technique for large, ptotic breasts eligible for BCS. The inverted-T scar technique has the advantage of a wide operative field, and the residual breast tissue or deepithelialized dermal tissue can be reconstructed by transposition in the desired direction with a relatively reasonable margin. Therefore, when the balancing procedure is performed simultaneously, it allows the surgeon to accurately inspect the affected area on the breast undergoing oncoplastic surgery, and to utilize every flap available to create a breast with the most natural shape possible. At the same time, in the intraoperative field, a similar amount and range of excision are determined by comparing the size and shape of the original breast and the excision amount during surgery. Intraoperatively, the sizes of both breasts and the projection and the degree of ptosis are adjusted and completed with a symmetric scar in the sitting position. However, radiation therapy can trigger dermatitis, fibrosis, scar formation, and seroma buildup; thus, it has been reported that radiation therapy is linked to unpredictable volume changes within 1 year after completion of radiation therapy, which affect the cosmetic outcomes of breast reconstruction [5,7,8]. Therefore, in order to create optimal symmetry, it is essential to analyze the predicted volume change, and to control the appropriate volume of the contralateral breast accordingly.
In patients who have undergone breast reconstruction using an extended latissimus dorsi muscle flap decreased latissimus dorsi muscle mass after radiation therapy is frequently observed due to factors including muscle atrophy and fibrosis [9,10]. However, in BCS, as muscle tissue is not used for breast reconstruction, it is predicted that the amount of volume decrease would be relatively small. In general, when performing balancing reduction (or mastopexy) of the contralateral breast after BCS, the affected breast is made to be slightly larger than the contralateral breast. In the procedures performed by the author, the majority of the patients showed reduced volume in both breasts more than a year after radiation therapy completion [9]. However, there were also many cases with different results. In some cases, the size of the radiation-treated breast did not become smaller as expected, and often remained slightly larger than the contralateral breast. Therefore, this study was conducted to examine whether breast volume would change significantly if both breasts are reconstructed to have similar intraoperative volumes by comparing volume changes between breasts that received BCS and the contralateral breasts that did not receive radiation therapy.
Patients were encouraged to start exercising when the sensation of heat and redness had disappeared after the radiation therapy. In general, upper body muscular workouts were carried out gradually starting 6 months after radiation therapy, and whole-body workouts such as swimming were encouraged starting 1 year after treatment. The degrees of swelling in both breasts was different, which frequently created a temporary asymmetry, but most of the swelling disappeared before radiation therapy.
No significant postoperative change was found in volume symmetry between both breasts after single-breast radiation therapy (P=0.97). That is, although the sizes of both breasts changed with other factors, such as weight loss, few cases showed asymmetry due to volume reduction after radiation therapy in comparison with the contralateral breast. Needless to say, uneven contracture deformities remain after radiation therapy in some situations, such as in patients with a very thin residual skin flap during surgery, those who undergo intensive excision in the inferior or internal area of the breast, or in patients for whom the excision range is too large in relation to the breast size. In these cases, the shape of both breasts appears to be similar immediately after surgery, but that the deformity progresses gradually for at least 1 year after radiation therapy. Except for those specific circumstances, it is thought that radiation therapy is unlikely to cause asymmetry, even if the breast is reconstructed to a similar size as that of the contralateral breast, and it is not necessary to reconstruct the affected breast to be larger based on the predicted effects of radiation therapy.
After surgery, it was observed that the patients’ weight frequently changed, resulting from postoperative hormonal therapy, patient’s individual activity, and occupational factors. As in breast cosmetic surgery, when body weight changes, the breasts may appear to be have relatively different sizes. Therefore, different size changes in both breasts may be observed after radiation therapy. In this study, it was observed that the breast treated with radiation became smaller in some patients, and that the contralateral breast that did not receive radiation therapy sometimes became larger. In general, however, the radiation-treated breasts did not become smaller than expected. Moreover, the results changed minimally even during a follow-up period of more than 2 years. Therefore, since there was no statistically significant difference in breast volume on both sides, and the size changes for both breasts showed a similar relationship with weight modifications, it can be argued that it is not necessary to reconstruct one breast to be larger than the contralateral side during BCS.
In cases of fat necrosis, the patient can feel the lump on the reconstructed breast, and may complain of discomfort or present with fear of breast cancer recurrence. This occurs particularly often with thin residual skin flaps. Furthermore, severe partial uneven deformation and deviation of the nipple areolar complex may appear after radiation treatment. In addition, since follow-up CT, ultrasonography, or magnetic resonance imaging can differentiate it from breast cancer recurrence, the possibility of small areas of fat necrosis must be clearly explained to the patient. Unlike patients who undergo breast cosmetic plastic surgery, patients with breast cancer who undergo breast reconstruction often have passive attitudes towards fat necrosis removal. Furthermore, unless they experience pain or discomfort, they usually do not want secondary surgery to modify the overall shape of the breast.
Despite the measures taken to reduce bias, such as limiting the cases analyzed to those with a follow-up period exceeding 2 years, a single operator, and identical surgical methods, radiation therapy, and CT, this study has the limitation of analyzing only 36 cases, which may not be sufficient for statistical significance. In addition, there were various amounts and ranges of excision in patients with asymmetric breasts, and their surgical results may have been affected by the surgeon’s experience. However, most of the surgical procedures in this study were performed with a minimal breast excision range and amount compared to the overall breast size. Therefore, this study is expected to be helpful for reconstruction surgery in patients who are concerned about the size of both breasts.
In a comparison of volume changes in both breasts using CT scans taken preoperatively and between 12 months and 18 months after radiation therapy, no statistically significant difference in volume was found between both breasts after BCS with an immediate balancing procedure with the inverted-T scar technique and single-breast radiation therapy. This suggests that it is not necessary to reconstruct the radiation-treated breast to be larger than the contralateral breast. In addition, in patients requiring such surgical treatment, it can be considered that asymmetry is unlikely to occur, even when immediate balancing is performed.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2020-123) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: MW Kim, SB Nam, JH Joo. Data curation: MW Kim, WS Oh, SB Nam. Formal analysis: KS Choo, SH Bae, C Kim, SB Nam. Methodology: WS Oh, KS Choo, KJ Nam. Project administration: MW Kim. Visualization: MW Kim, YJ Jung, KS Choo, KJ Nam, SH Bae. Writing-original draft: JW Lee, JH Joo. Writing-review & editing: WS Oh, JW Lee, HY Kim, YJ Jung, KJ Nam, C Kim.