Management of keloid scars: noninvasive and invasive treatments
Article information
Abstract
Scars vary from mature linear scars to abnormal excessive scars such as hypertrophic scars and keloid scars. Keloid scars are fibro-proliferative disease entities that reflect an abnormal process of wound healing. They can cause pain, itching, stiffness, and psychological distress, all of which can affect quality of life. Various treatment options have been advocated as ways to prevent and treat keloid scars. These include noninvasive treatments such as use of silicone gel sheeting and compression therapy, and invasive treatments such as intralesional corticosteroid injections, surgery, and radiotherapy. Novel treatments include chemotherapy, immunotherapy, and anti-inflammatory therapies. Unfortunately, keloids continue to pose a significant challenge due to the lack of efficacious treatments. Therefore, clinicians should be familiar with various therapeutic options and apply the most suitable treatment plan for patients. In this review, we introduce the current therapeutic options for the management of keloid scars.
INTRODUCTION
Scars vary from mature linear scars to abnormal excessive scars such as hypertrophic scars and keloid scars. It is estimated that about 100 million people worldwide have acquired scars from surgery or trauma, of which 15% of scars are excessive [1].
According to the classification proposed by Mancini and Peacock, excessive scarring is divided into hypertrophic or keloid scarring [2]. Both types of scars rise above the skin level. However, the extent of hypertrophic scars is most commonly limited to the area of the original wound, and they sometimes spontaneously regress; in contrast, keloid scars extend beyond the boundaries of the original wound, and continue to invade the adjacent normal skin while remaining elevated [1-8]. Keloid scars are fibro-proliferative disease entities that reflect an abnormal process of wound healing [3,9,10]. Their cause is unknown, but both genetic and environmental factors are involved [9,11].
The prevalence of keloid scars ranges from 0.09% in the United Kingdom to 16% in the Congo, with equal frequencies in both sexes [5,8,12]. Although keloid scars can occur at all ages, their incidence is high in the second and third decades of life, and injuries in young adults seem to produce more severe keloids than those in elderly people [2,3,5,9,12]. Individuals with pigmented skin are more likely to develop keloids [6], and Hispanics, African-Americans, and Asians all have an elevated risk of forming keloid scars, with a prevalence of keloid scars of 10% in the African-American population [2,3,7,9,11]. Other known risk factors are type A blood, hyper-immunoglobulin E syndrome with a high risk of allergy, and hormonal peaks such as puberty and pregnancy [11,13,14]. Keloids most frequently occur in body areas that are under tension, such as the lower abdomen and the deltoid, sternal, and suprapubic regions [6,7]. Other common locations are the shoulders, earlobes, and all areas that lack hair follicles and glands [3].
Keloid scars are not only a type of physical and aesthetic impairment, but also have psychological and social sequelae, which can further impair patients’ quality of life [11,15]. They can cause significant pain, persistent itching, stiffness, and scar contracture [1,5,12,15]. In addition, they can have psychological effects, including reduced self-esteem, disruption of daily life, anxiety, and depression [1,5,12,15,16].
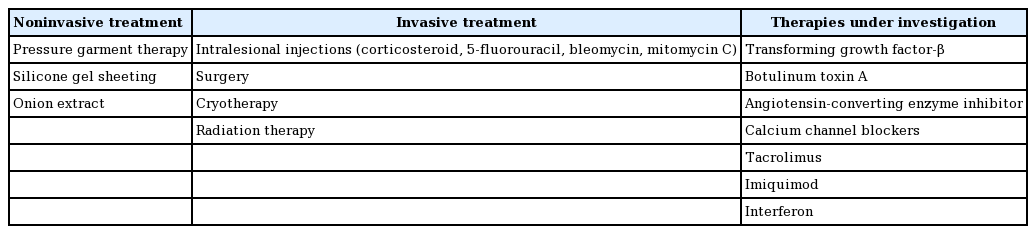
Various treatment options have been proposed as ways to prevent and treat keloid scars. Although differences might exist in the pathogenesis of keloid scars across ethnicities, the international advisory panel, Chinese expert consensus, and Japan scar workshop consensus have presented similar treatment options [1,17-21]. These include noninvasive treatments such as silicone gel sheeting, physiotherapy, and compression therapy, as well as invasive treatments such as intralesional corticosteroid injections, radiotherapy, cryotherapy, and surgery (Table 1) [1,17].
Despite the introduction of numerous available treatment options, keloid scars remain a therapeutic challenge. Therefore, clinicians should be familiar with the therapeutic options and to be able to provide the most suitable treatment. Here, we introduce the currently available therapeutic options and strategies, both noninvasive and invasive, for the management of keloid scars, as well as novel therapies under investigation.
NONINVASIVE TREATMENTS
Pressure garment therapy
In the past 45 years, pressure therapy was not only an option for treating keloid scars, but also the standard first-line treatment for burn scars [3,22-25]. However neither its underlying mechanism of action nor evidence of its effectiveness has been investigated [3,7,26].
Pressure therapy can be initiated after wound closure and when the patient can tolerate the pressure [18]. It requires specially-fitted garments that should be worn at least 23 hours per day. The recommended pressure is 24–30 mmHg, and the length of treatment should be 6–24 months [3,7,11,23].
Pressure garment therapy alleviates itching and pain, but its disadvantages are the cost and poor patient compliance due to the significant discomfort caused by the garments [23,27]. Furthermore, a meta-analysis reported that the potential morbidity and costs of pressure therapy appear to outweigh its benefits [11,28]. Children with keloids may be treated with pressure therapy, since the side effects are minimal compared to other invasive therapies [7].
Silicone gel sheeting
Silicone materials have been recommended as the “gold standard” treatment of keloid scars [1,29]. Their effects are thought to be related to occlusion and hydration [11,30]. Studies have reported up to 90% improvement of keloid scars following the use of silicone dressings [22]. However, although silicone materials decrease the incidence of keloids after surgical procedures, complete resolution has not been described [24,31]. Furthermore, the use of silicone materials lacks a scientific underpinning and well-designed studies [11].
Silicone materials have been manufactured as sheets and gels, which have shown equivalent efficacy [32]. It is recommended that patients wear silicone sheets for 12–24 hours per day for 3–6 months. Silicone gels should be applied twice daily [1,7].
No serious side effects have been reported, but folliculitis is a potential adverse effect [11]. Another disadvantage is that most of the available products are expensive [7].
Onion extract
The primary component of onion extract is quercetin, a flavonoid known for its anti-inflammatory, antibacterial, and collagen-suppressive properties [33]. Quercetin inhibits fibroblast proliferation and collagen production and thus reduces excess scar formation [7,34]. It also has an antihistamine effect, which is relevant for keloids since histamine increases collagen production by fibroblasts, and has therefore been implicated in the formation of keloids [35,36]. However, the exact mechanism through which onion extract reduces scar formation remains to be clarified [3,37].
INTRALESIONAL INJECTIONS
Intralesional corticosteroid injections
Current international guidelines recommend intralesional corticosteroid injections as the first-line therapy for the prevention and treatment of keloids [18,19,38]. They can be used alone or in conjunction with other treatments [30].
Corticosteroids exert their effects by decreasing fibroblast proliferation, reducing collagen synthesis, altering extracellular matrix components such as glycosaminoglycan, and repressing inflammation [30,39]. The decreased collagen synthesis is thought to be due to fibroblast hypoactivity, reduced fibroblast density, and modification of fibroblast maturation [30,40]. Intralesional corticosteroid injections improve scar pliability, diminish scar volume and height, and lead to rapid clinical improvement of associated symptoms such as itching and pain [7,11,41].
Triamcinolone acetonide is used clinically at concentrations between 10 and 40 mg/mL, with 2 to 3 injections per month administered for 6 months or even longer, depending on the location, size, and volume of the keloid, as well as the individual patient’s characteristics [6,39,42]. Although injections are painful, the response rate ranges from 50% to 100% and the recurrence rate is 9% to 50% [6,7,32,42]. The results can be improved by combining the injections with other treatments, such as surgery, 5-fluorouracil, and cryotherapy [11,13,22,38]. When injections were employed as adjuvant therapy after surgery, recurrence rates varied from 1% to 100%, but were less than 50% in most studies [30].
The side effects of intralesional injections are pain, skin atrophy, hypopigmentation, hyperpigmentation, and telangiectasia [6,42,43].
5-Fluorouracil
Since keloids display a cellular hypermetabolic state, antineoplastic agents have been considered as a reasonable form of therapy. 5-Fluorouracil has been employed as a treatment option for keloids for more than 25 years, but its use remains controversial [44].
5-Fluorouracil is a fluorinated pyrimidine analogue and a classical chemotherapeutic agent [30,45]. It functions as a cytotoxic agent, inhibiting cell proliferation in the scar tissue [7,44,45], and has been shown to inhibit fibroblast proliferation and enhance fibroblast apoptosis without causing tissue necrosis. It also inhibits transforming growth factor-β (TGF-β)–induced expression of type I collagen [46-48].
Intralesional 5-fluorouracil has been used alone, in conjunction with corticosteroids, or as adjuvant therapy after surgery [1,7,30]. It was effective in 45%–96% of patients, and yielded better results when combined with corticosteroid treatment [46]. The recommended ratio is 9:1 (0.9 mL of 50 mg/mL 5-fluorouracil: 0.1 mL of 40 mg/mL triamcinolone) monthly, if corticosteroid injections have failed or in particularly severe cases [7].
The side effects of 5-fluorouracil include pain, burning sensation, purpura formation, temporary hyperpigmentation, skin erythema, and ulceration [3,7,13,41,48]. However, intralesional 5-fluorouracil treatment is safe, and no systemic complications such as anemia, leukopenia, or thrombocytopenia have been reported [3,49].
Bleomycin
Bleomycin is a cytotoxic anticancer agent with antibacterial and antiviral activities [49-51]. It induces apoptosis and reduces TGF-β1-induced collagen synthesis [30,51-53].
Intralesional injections of bleomycin start at 0.1 mL (1.5 IU/mL) and can be increased to a maximum dose of 6 mL, with two to six sessions per month for keloids that are unresponsive to intralesional corticosteroid injections [3,53].
Side effects include pain, superficial ulceration and crusting at injection sites, transient hyperpigmentation, and dermal atrophy [49,54]. No systemic toxicity, such as pulmonary, renal, cutaneous, hepatic, or myelogenous toxicity, has been reported for low-dose subcutaneous injections of bleomycin [53].
Mitomycin C
Mitomycin C is a derivative of Streptomyces caespitosus, and has antineoplastic and anti-proliferative activities [25]. It inhibits the synthesis of DNA, RNA, and protein. It also inhibits fibroblast proliferation and prevents cell division, thereby reducing scar formation both in vitro and in vivo [53,55]. There is increasing interest in the use of mitomycin C to treat keloid scars [3].
SURGERY AND OTHER INVASIVE TREATMENTS
Surgery
Surgical excision of keloids is a popular option and is recommended as the first-line treatment if disabling scar contracture is present [3,56]. However, it should be used with caution since it often creates even larger lesions, and recurrence rates are high (45%–100%) [25,57]. Adjuvant measures, such as radiotherapy, interferon, bleomycin, cryotherapy, or corticosteroids, should be applied to avoid recurrence [11,22,31]. For example, combining corticosteroid treatment with surgery reduced the recurrence rate to less than 50%, and the recurrence rate for surgery with adjuvant radiotherapy ranged from 0% to 8.6% [25,58,59].
As a general rule, wound closure should be performed with minimal tension and sutures, and relaxed skin tension lines, leaving everted wound borders [30,56,60]. In cases of scar contracture caused by excessive tension, Z-plasty, W-plasty, or various local flaps may be indicated [56,60].
Cryotherapy
Cryotherapy leads to cellular injury and necrosis of keloid tissue. It can be administered by contact, spray, or intralesional injection [30]. Intralesional cryotherapy concentrates the area of cold within the lesion, thereby minimally affecting the external skin; it is simple, can be applied to all types of scars [3], and is more effective than contact/spray treatment [14,61].
Cryotherapy is applied monthly in multiple sessions, and the success rate after two sessions ranged from 30% to 75% [11,45]. Cryotherapy, in combination with intralesional corticosteroid injection, has been the most popular traditional treatment for keloids [3,11,41].
The most common side effect of cryotherapy is hypopigmentation, followed by blisters, local pain, and hyperpigmentation [3,7,30,62].
Radiation therapy
Radiation therapy is most effective as an adjunct to surgery [1,3]. The combination of radiation therapy and surgery was found to be the most effective treatment in severe keloid cases [11] and reduced recurrence by 55% at 30 months of follow-up [3]. The exact mechanism of action of radiotherapy is unknown [30]. It may act by inhibiting the proliferation of fibroblasts, preventing fibroblast repopulation, or inhibiting angiogenesis [63-66].
The best results can be achieved with 15–20 Gy over five or six sessions during the early postoperative period (24–48 hours after surgery) [11,41]. To reduce recurrence and simultaneously limit complications, radiotherapy is applied using a site-dependent dose. For keloids on the anterior chest wall, scapular region, or suprapubic region, 20 Gy is given in four fractions over 4 days; for earlobe keloids it is given as 10 Gy in two fractions over 2 days, and for keloids at other sites, 15 Gy in three fractions over 3 days [8,64].
The side effects of radiotherapy include acute skin reactions, such as desquamation, epilation, pigmentation, and erythema in the early period (7–10 days), while subacute and late complications include scarring, permanent pigmentation, atrophy, depigmentation, telangiectasia, subcutaneous fibrosis, and necrosis several weeks later [8,17].
Radiation therapy is not recommended for pregnant patients, children (less than 12 years old), or for keloids in radiosensitive locations (such as the thyroid) [30]. Concerns have been raised regarding the risk of developing cancer. Overall, the evidence suggests that cancer development on keloid scars post-radiation therapy is rare [13,67,68].
THERAPIES UNDER INVESTIGATION
Transforming growth factor-β
The TGF-β family contains several isoforms (e.g., TGF-β1, -β2, and -β3) that are strongly implicated in the scarring process [2]. For example, fetal healing with no scarring was associated with a higher ratio of TGF-β3 to TGF-β1 and TGF-β2. In addition, oral wounds that healed more rapidly without scars also had a higher ratio of TGF-β3 to TGF-β1 [69,70]. Therefore, TGF-β3 can be considered to have anti-fibrotic properties, whereas TGF-β1 and TGF-β2 contribute to scarring [30,31].
A recent double-blind, placebo-controlled study of recombinant human TGF-β3 (Avotermin) resulted in a significant reduction of scarring. Avotermin has been shown to be safe, with little systemic effect; its side effects were erythema and edema [71].
Botulinum toxin A
The use of botulinum toxin A reduces tension on the wound edges by preventing muscle contraction during the healing process, thereby reducing scar formation [9,30,72]. In fact, tension is one of the causes of keloid scars. Botulinum toxin A is given 4 to 7 days before surgery to reduce tension [17].
Intralesional botulinum toxin injections resulted in improvement of keloids in a prospective, non-controlled study [49,73], and decreased keloid volume more effectively than intralesional corticosteroid injections [49]. However, conflicting results have been reported for the therapeutic effects of botulinum toxin [8,74]. Larger, randomized, controlled studies are needed to confirm its efficacy [11].
Angiotensin-converting enzyme inhibitor
The renin-angiotensin-system has been shown to affect collagen production and wound healing [3]. Although it is still under study, the local application of captopril cream (5%) and oral administration of enalapril improved keloids with no side effects. Therefore, these may be good treatment options for keloids [41,75,76].
Calcium channel blockers
Verapamil is a calcium channel blocker that is used as an antihypertensive agent. The mechanism of action of calcium channel blockers on keloids is assumed to involve reduced levels of intracellular calcium [77], leading to increased collagenase synthesis and ultimately, reduced scar tissue [3,78].
Application of verapamil cream to keloid scars prevents recurrence after intralesional injections, and intralesional verapamil injection after surgery also increased the cure rate [79-81]. However, other studies showed no evidence for the efficacy of verapamil [82,83]. These mixed results indicate that further study research is necessary on the topic of verapamil therapy in keloid treatment.
Tacrolimus (FK-506)
Tacrolimus is a calcineurin inhibitor and an immunosuppressive medication [8,30]. When it was applied to keloid fibroblasts in vitro, it reduced their proliferation, migration, and collagen production [8,84]. Clinically, tacrolimus is used as a topical medication for dermatological conditions such as atopic dermatitis. One patient using tacrolimus for treatment of atopic dermatitis reported that it also reduced keloid scarring [85]. However, further research on the efficacy of tacrolimus is needed [30].
Imiquimod
Imiquimod is an immune response modulator [41,86,87]. As a Toll-like receptor agonist, it increases the production of pro-inflammatory cytokines tumor necrosis factor-α, interferon-α, and interleukin 1, 6, 8, and 12. Furthermore, it induces the expression of apoptotic genes in keloid tissue [8,14,30,41,86,88].
Imiquimod cream at a 5% concentration has been approved for treating warts, basal cell carcinoma, and actinic keratosis [13,25]. A meta-analysis demonstrated that the estimated keloid recurrence rate in patients who received adjuvant therapy with imiquimod cream after keloid surgery was 24.7% [55]. Although many clinical studies point to the effect of imiquimod in the prevention of recurrence after keloid surgery, its efficacy is still open to question [86,89].
Its reported side effects are pain, hyperpigmentation, and local skin reactions such as irritation, erythema, erosion, and crusting [30,90].
Interferon
Interferons are cytokines with antiproliferative, antifibrotic, and antiviral effects [51,91]. They have also been shown to increase collagen breakdown [22,92]. Although they have promising effects on keloids, interferon treatment is expensive and remains controversial [8,11,13,57]. Moreover, interferon injections are painful and may require regional anesthesia [11,92].
Adverse effects are generalized flu-like symptoms (occurring in 73.7% of subjects), and pain and inflammation at the injection site [30,93,94].
Tamoxifen
Tamoxifen is a synthetic nonsteroidal anti-estrogen used to treat breast cancer [8,30]. It also has an antifibrotic effect, with success in treating retroperitoneal fibrosis and desmoid tumors [95,96].
It has been shown to inhibit proliferation of cultured keloid fibroblasts, and to decrease collagen synthesis by these fibroblasts, by lowering TGF-β1 production [97,98]. However, further studies are needed to determine the potential impact of this medication on keloids.
AN ALGORITHM FOR THE MANAGEMENT OF KELOID SCARS
An international, multidisciplinary group composed of 24 experts (including dermatologists, plastic surgeons, general surgeons, medical, rehabilitation and burn specialists, psychosocial and behavioral researchers, epidemiologists, and beauticians) recently developed a set of practical guidelines for the prevention and treatment of linear, hypertrophic, and keloid scars [1,22].
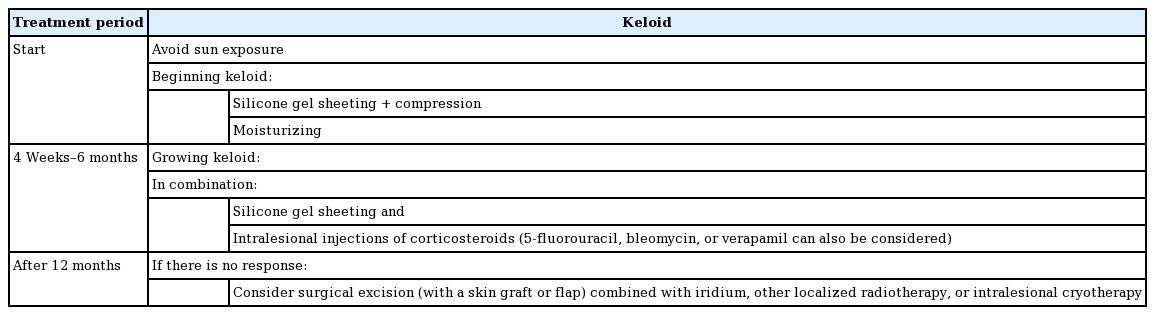
For keloids, the primary treatment recommended was silicone gel sheeting with compression therapy and moisturizing. If keloids continue to grow, silicone gel sheeting and pressure therapy, as well as intralesional injections of corticosteroids (5-fluorouracil, bleomycin or verapamil can also be considered) are recommended in combination. If there is no response, surgical excision (along with a skin graft or flap) may be considered, combined with iridium, other localized radiotherapy, or intralesional cryotherapy (Table 2) [1,17].
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.