Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
Article information
Abstract
In the field of tissue engineering and reconstruction, the development of efficient biomaterial is in high demand to achieve uncomplicated wound healing. Chronic wounds and excessive scarring are the major complications of tissue repair and, as this inadequate healing continues to increase, novel therapies and treatments for dysfunctional skin repair and reconstruction are important. This paper reviews the various aspects of the complications related to wound healing and focuses on chitosan because of its unique function in accelerating wound healing. The proliferation of keratinocytes is essential for wound closure, and adipose-derived stem cells play a significant role in wound healing. Thus, chitosan in combination with keratinocytes and adipose-derived stem cells may act as a vehicle for delivering cells, which would increase the proliferation of keratinocytes and help complete recovery from injuries.
INTRODUCTION
The skin is the protective, defensive barrier from the outside world; therefore, any break or injury in the skin should be efficiently mended. Wound-healing biology involves the signaling pathways that trigger relatively inactive cell lineages to the wound margin for the proliferation, invasion and reconstruction of new matrices in the wound gap [1]. Studies have established that the incidence of severe burns in the United States is estimated to be 70,000 per year [2]; the occurrence of venous leg ulcer is between 600,000 and 1,500,000 [3]; and the prevalence of chronic foot wounds in people suffering from diabetes is 15 to 20 percent [4]. Thus, the dressing cost alone for the above-mentioned cases has been calculated as $5 billion per year [5]. Although multiple treatments such as skin grafting and skin reconstruction with biomaterials exist, further development and refinement of skin substitutes or biomaterials are essential for perfect skin reconstruction.
WOUND-HEALING PROCESS
Wound healing is a complex mechanism that begins with an inflammatory phase followed by re-epithelialization and ending with a remodeling phase. Tissue injury disrupts vascular vessels and initiates extravasation. The inflammatory response begins with the release of growth factors, cytokines and components of extracellular matrices (ECM). Then, the tissue re-epithelialization phase, which is a process mainly involving the migration and proliferation of keratinocytes to resurface the wound area with a layer of new epithelium, takes place. As this epidermal layer continues migrating, the keratinocytes at the wound margin begin to proliferate and migrate to contact the wound margin [1,6,7,8]. Finally, the remodeling phase, where the collagen fibers reorganize and mature to gain tensile strength, occurs [8]. Thus, the wound-healing mechanism is a complex chain of events involving different cell-to-cell interactions and interactions among tissues that are impaired due to a number of medical conditions. Skin injuries could be healed more quickly, but re-epithelialization is not always perfect and leaves a connective tissue scar. Therefore, the study of the proliferation of keratinocytes, which plays a major role in re-epithelialization, is important for tissue reconstruction.
SKIN SUBSTITUTES FOR WOUND HEALING
The initial treatments for severe injuries and burn cases include autografts, allografts and xenografts. However, these treatments often have limited donor sites. Recently, studies have shown that bioengineered skin substitutes are an advancement in tissue engineering with a wide range of applications in wound healing [5]. Based on the depth of the injuries, wounds can be classified into epidermal, superficial partial thickness, deep partial thickness and full thickness [9]. Therapeutic approaches for the treatment of deep dermal and full-thickness injuries remain unsatisfactory; therefore, more effective treatment strategies are needed [10].
In cases of severe burn injuries, a stretched, meshed skin graft could be used. The focus has then moved to skin substitutes, and the main goal behind this method is to accelerate wound healing using the normal repair mechanism, provide a surface for cells to proliferate and prevent bacterial infection. Based on the requirements of skin injury, different types of skin substitutes are used for specific purposes [11,12,13,14,15,16,17,18,19,20]. Table 1 shows some of the widely used skin substitutes [18,19].
These bioengineered skin substitutes can offer four functions such as protection: creating a defense barrier to micro organisms, procrastination: to achieve permanent wound closure particularly in case of extensive burn injuries, promotion: delivering matrix components, growth factors and cytokines, provision: incorporation of dermal collagen or cultured cells at the wound site [21]. Although the meshed skin graft covers a greater area, most of the skin grafts and skin substitutes that has been used have various disadvantages, such as slow epithelialization, delayed wound healing, graft contraction, scarring of tissues, slow vascularisation and inadequate acceleration of wound healing [22].
Additionally, skin substitutes should be cost effective, readily available, resistant to infection and have a longer shelf life. Unfortunately skin substitutes with all of these properties are unavailable in the market. Because of the great importance and high demand of skin substitute products, research should be carried out to develop an ideal skin substitute [18].
CHITOSAN IN WOUND HEALING
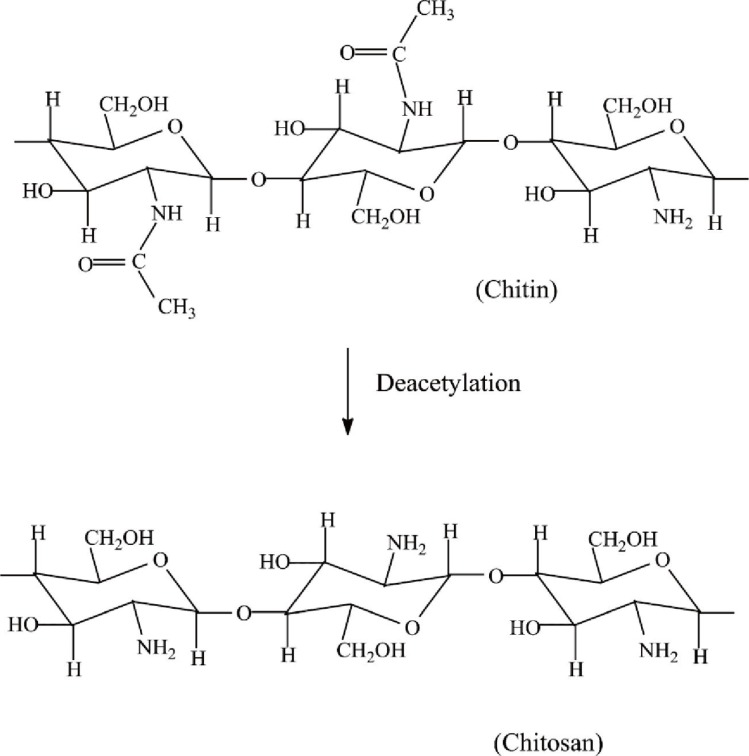
Chitosan is an abundantly available biopolymer composed of (1-4)-2-acetamido-2-deoxy-b-D-glucan (N-acetyl D-glucosamine) and (1-4)-2-amino-2-deoxyb-D-glucan (D-glucosamine) units, which are partially derived from the deacetylation of chitin polymers [23]. Chitin is most commonly found in invertebrates such as crustaceans or insect cuticles, mushrooms, green algae, yeasts and the shells of shrimp and crab [24,25,26,27]. However, the poor solubility of chitin limits its practical usage. The presence of amino groups differentiates chitosan from chitin and gives unique properties to the chitosan polymer, which has more clinical and non-clinical applications (Fig. 1) [28,29].
Chitosan biopolymers have been widely used in the fields of biotechnology, cosmetics, biomedicine, food and agriculture [26]. In tissue engineering, chitosan that accelerates wound healing has been used for wound dressings and creating artificial skin [27]. Chitosan is found to be biodegradable and biocompatible and is an excellent hemostatic and analgesic agent with antioxidant properties [26]. Chitosan enhances the functions of inflammatory cells and growth factors, thereby promoting granulation and remodeling of damaged tissues in large, open wounds of animals [30]. It has been shown that chitosan hydrogel interacts with fibroblast growth factor (FGF-2) on an open-wound surface in a mouse model. This interaction resulted in contraction of the wound, formation of granulation tissue, closure and healing of the wound [31]. Recent studies have shown that a bilayer chitosan membrane, which consists of an upper chitosan film layer attached to an inner layer of porous membrane, serves as an efficient skin-regenerating template for treating third-degree burns and cutaneous wounds. This chitosan bilayer has the potential to enhance the proliferation of fibroblasts, thereby forming a monolayer to cover the wound surface [32]. The three dimensional structural organization of chitosan is essential to serve as a vehicle for delivering and retaining the cells at a specific site and to initiate appropriate cell-to-cell interactions [33]. Chitosan supports the adhesion and activation of platelets, which are enhanced by plasma and extracellular matrix proteins [34]. Based on the literature, tissue-engineering scaffolds should 1) be biodegradable to favor the cured tissue in replacing the biomaterial, 2) not trigger the acute or chronic inflammatory responses, 3) have surface properties that enhance the attachment, proliferation and differentiation of cells, 4) mimic the skin in vitro, 5) have suitable mechanical properties, and 6) be suitable for manufacturing into different shapes [28]. Chitosan when compared with the above mentioned skin substitutes, has all of these remarkable properties which make chitosan scaffold as a promising future in the management of wound healing. Also, its availability in different forms would serve as efficient scaffolds in the treatment of acute and chronic wound injuries.
KERATINOCYTES AND CHITOSAN
Keratinocytes are the predominant cell components of the epidermis. These cells play a significant role in the wound-healing process because they are involved in the complex mechanisms of initiation, proliferation and re-epithelialization of wound healing. Normal and healthy keratinocytes differ from the keratinocytes at the non-healing chronic wound edges. In cases of injuries, the migration of basal keratinocytes from the wound margin and cut epidermal appendages to the denuded wound surface are essential to carry forward or move over the newly reconstructed dermal scaffolding. The stratified keratinocytes proliferate and differentiate to produce neoepidermis, which covers the entire wound surface and restores the skin function [35]. For the successful closure of wounds, the proliferation of keratinocytes is essential to facilitate communication with other cell types that are involved in wound healing [36].
Because chitosan has been employed as the best biomaterial for wound dressing, studies have been conducted to determine the interactions between chitosan and keratinocytes. The degree of acetylation (DA) is a term used to define chitin and chitosan. DA is an essential structural parameter that influences biological and wound-healing properties [37,38,39]. Cultures of keratinocytes were analyzed on five different chitosan films with DAs ranging from 2.5% to 47%, and the cell adhesion and proliferation of the keratinocytes on these chitosan films were investigated. The DA does not influence the cytocompatibility of chitosan films with keratinocytes in vitro; however, fully deacetylated chitosan films allowed for better adhesion to keratinocytes, resulting in their better proliferation. This finding indicates that chitosan films with low DA could act as efficient biomaterials because they would adhere to fibroblasts and induce the proliferation of keratinocytes and re-epithelialization [40].
The effects of chitin and chitosan on the proliferation of keratinocytes in vitro were studied. Primary human keratinocytes and an immortalized human keratinocyte cell line (HaCaT) were cultured with and without an irradiated fibroblast feeder layer. Chitosan and the primary keratinocytes with the irradiated feeder layer supported the growth and proliferation of keratinocytes in vitro [41]. This study proves that highly deacetylated chitosan has more potential for wound healing. However, the mechanism of interaction between chitosan and keratinocytes is not clear [42]. Hence it is important to study the proliferation of keratinocytes on chitosan scaffold.
ADIPOSE-DERIVED STEM CELLS AND CHITOSAN
Current research findings reveal that bone marrow-derived stem cells (BMSCs) were found to play an important role in tissue repair. BMSCs secrete growth factors that enhance the stimulation, proliferation and regeneration of damaged cells [43]. The transplantation of BMSCs require harvesting of large numbers of bone marrow cells under general anesthesia, which leads to several complications that limit its use [44,45]. Adipose-derived stem cells (ASCs) are pluripotent stem cells that are derived from adipose tissue and have characteristics similar to mesenchymal stem cells derived from bone marrow. ASCs were found to have potent applications in the repair and regeneration of damaged tissues by helping in wound healing and in treatment for scarring and photoaging. Therefore, ASCs can be used therapeutically to treat chronic wounds and other conditions. However, for massive tissue damage, the available ASCs are insufficient to efficiently repair the damaged tissue, but this could be overcome by using adipose tissue obtained from liposuction procedures [46,47]. These ASCs, when combined with chitosan, have been found to increase the repairing and healing potential of damaged tissues. The microenvironment for skin regeneration mainly depends on interactions between stem cell progenitors and their niche [48]; therefore, any tissue-engineered reconstruct should provide a suitable microenvironment for the cells to proliferate and differentiate.
ASCs were seeded on silk fibroin chitosan (SFCS), and the impact of ASC-SFCS on wound healing was evaluated. Because of its biocompatible nature, silk fibroin was hybridized to chitosan, and the resulting hybrid matrix mimicked the constituents of the extracellular matrix and served as a substrate for cell adhesion and migration and the incorporation of tissues [49,50]. In a murine cutaneous injury model, ASC engrafts proliferated and differentiated into vascular, fibroblastic and epithelial cell phenotypes in their newly established microenvironment. The ASCs-SFCS also showed vascular enhancement, and SFCS acted as a delivery vehicle that provided a supportive niche for the migration, proliferation and differentiation of the cells. This study elucidated that ASC-SFCS supports the engraftment of stem cells and their differentiation into epithelial and fibrovascular components; therefore, it could be applied in the clinical setting [51]. It has also been found that ASCs induce osteogenic and chondrogenic differentiation in chitosan-agglomerated scaffolds, which are used as substitutes for the ECM [52]. In a similar study, human Hair Follicle Stem Cells (HFSCs) combined with fibroblasts were seeded in chitosan and found to be successful in accelerating wound healing in full-thickness wounds of irradiated rats [53]. Studies also revealed that ASCs could enhance the proliferation of HaCat cells (immortalized human keratinocytes) and accelerate in vitro wound healing [54]. Therefore, it is critical to study the proliferation of human keratinocytes that is enhanced by ASCs on a chitosan scaffold in vitro.
CONCLUSIONS
The unique properties of chitosan may increase the proliferation of keratinocytes when seeded with ASCs. It is therefore important to study the proliferation of human keratinocytes induced by ASCs using the in vitro model of the chitosan scaffold. This scaffold could help maximize the healing potential, for proper skin regeneration. Research should be carried out to establish the stimulatory effects of ASCs on a chitosan scaffold and to determine the cellular and molecular mechanisms involved. Furthermore, importance should be placed on the efficient development of chitosan skin substitutes with long-term safety and longer shelf life. Thus, this review has highlighted the importance of chitosan in tissue reconstruction, which paves the way for novel therapeutic strategies.
Notes
The work related to this paper was supported by a Research University Grant (1001/PPSP/813058) from Universiti Sains Malaysia.
No potential conflict of interest relevant to this article was reported.