Intraoperative near-infrared spectroscopy for pedicled perforator flaps: a possible tool for the early detection of vascular issues
Article information
Abstract
Background
Pedicled perforator flaps can present postoperative complications similar to those encountered in free flap surgery. Beyond a clinical evaluation, there is still no reliable technical aid for the early prediction of vascular issues. The aim of this study was to assess the support of near-infrared spectroscopy technology as an intraoperative tool to anticipate postsurgical flap ischemia.
Methods
We prospectively enrolled 13 consecutive patients who were referred to our hospital from March 2017 to July 2018 and required a reconstructive procedure with a pedicled fasciocutaneous perforator flap. We measured flap peripheral capillary oxygen saturation (SpO2) in each patient with a Somanetics INVOS 5100C Cerebral/Somatic Oximeter (Medtronic), both before and after transposition. Patient demographics, operative data, and complications were then recorded during the following 6 months. We analyzed the data using the Wilcoxon signed-rank test and linear regression.
Results
The mean flap SpO2 before and after transposition was 92%±3% and 78%±19%, respectively. The mean change in SpO2 was 14%±17%, with a range of 0% to 55%. The change in saturation and mean saturation ratio were significantly different between patients with and without postoperative flap necrosis.
Conclusions
An immediate quantitative analysis of flap peripheral capillary SpO2 after transposition has never before been described. In our experience, an intraoperative drop in SpO2 equal to or greater than 15%–20% predicted vascular complications in pedicled perforator flaps. Conversely, flap size and rotation angle were not correlated with the risk of flap necrosis.
INTRODUCTION
Perforator flaps are a reliable reconstructive technique, with versatility and minimal donor site morbidity [1-3]. High mobility and ample transposition are clear advantages of perforator flap surgery. However, flap movement directly affects the small perforator pedicles. When used as pedicled flaps, venous congestion and partial necrosis have been reported to occur in up to 42% of cases [4,5].
The early detection of possible complications is related to the surgeon’s experience, and is essentially based on flap clinical monitoring, including skin color, turgor, swelling, capillary refill time, temperature, and bleeding characteristics after a pinprick [6-8]. All of these evaluations are made postoperatively, when little can be changed in the planned reconstruction.
Many new instrumental techniques have been proposed to support clinical monitoring of free flaps [9,10]. Near-infrared spectroscopy (NIRS)–based technology measures the capillary blood oxygen saturation (SpO2) in the dermal capillaries, immediately after its noninvasive application. A sterile probe can be used for intraoperative measurements. However, the use of NIRS in flap monitoring has only been reported in two animal studies. A decrease in SpO2 within hours after flap elevation was reported to predict flap viability [11,12].
The aim of this study was to use NIRS technology for the immediate evaluation of flap vascularization and to assess the ability of immediate NIRS to predict the postoperative clinical course.
METHODS
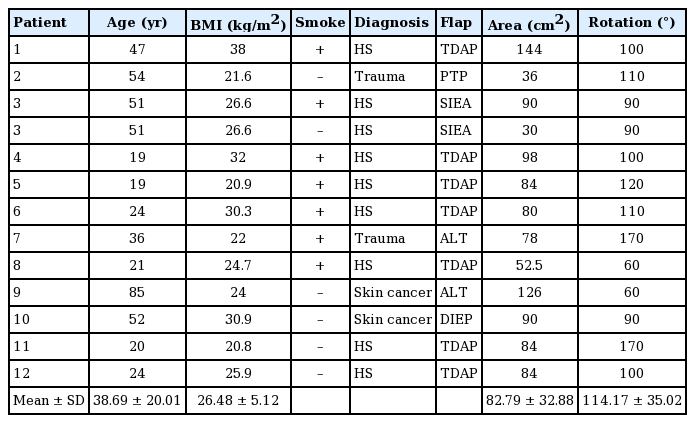
We prospectively analyzed 12 consecutive patients needing 13 pedicled perforator flaps for reconstructive surgery, who were referred to our department from March 2017 to July 2018. All patients were preoperatively studied with Huntleigh Doppler ultrasound for perforator vessel identification and mapping. Intraoperative flap superficial oximetry was recorded with Somanetics INVOS 5100C Cerebral/Somatic Oximeter (Medtronic, Dublin, Ireland) sterile patch sensors, both before transposition and after transposition of the flap to the definitive recipient site (Figs. 1, 2).
A patient who suffered from Hurley stage III hidradenitis suppurativa and was treated with wide local excision followed by immediate reconstruction with a thoracodorsal artery perforator (TDAP) flap. In the case shown here, we intraoperatively monitored the pedicled TDAP flap with near-infrared spectroscopy before its transposition into the axillary defect.
Postoperative monitoring of a pedicled thoracodorsal artery perforator flap with near-infrared spectroscopy after flap inset.
The change in SpO2 (ΔSpO2, expressed in percentage points) indicates the absolute intraoperative drop in SpO2. The SpO2 ratio, defined as (SpO2 before transposition–SpO2 after transposition)/SpO2 before transposition, refers to the relative intraoperative drop of SpO2 and allows a better comparison of results, independent from the absolute values of SpO2. The arc of rotation (in degrees) and area of harvesting (in square centimeter) were registered intraoperatively for every pedicled flap. In all cases, perforator identification, oximetry measurement, and flap dissection were performed by the same surgeon in the same department. The differences between oximetry changes were evaluated using the Wilcoxon signed-rank test. The association between flap saturation and necrosis occurrence was analyzed using linear regression analysis.
RESULTS
Seven male and five female patients were referred to our department for reconstruction with a perforator flap. Nine patients suffered from axillary Hurley stage III hidradenitis suppurativa. A thoraco-dorsal artery perforator flap was the most frequent reconstruction. Patients’ mean age was 38.7 ± 20.0 years, with a mean body mass index (BMI) of 26.5 ± 5.1 kg/m2. Seven patients were active smokers. Only one patient had diabetes mellitus. No other known comorbidities were present that would constitute a risk factor for the outcomes of perforator flap reconstruction. Details about the diseases and reconstruction techniques are outlined in Table 1.
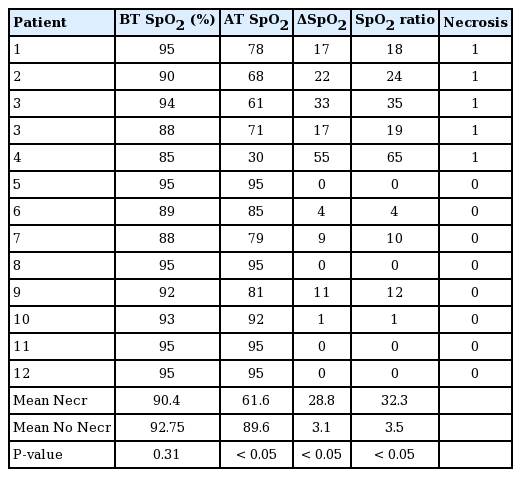
The mean flap area was 82.79 ± 32.88 cm2, with a mean rotation angle of 114.17° ± 35.02°. The mean flap SpO2 before and after transposition were 92% ± 3% and 78% ± 19%, respectively. The mean ΔSpO2 registered was 14% ± 17%, with a range of 0% to 55%. The mean SpO2 ratio was 15% ± 19%. Details about flap characteristics and oximetry measurements are outlined in Table 2.
Intraoperative flap superficial oximetry values before and after transposition of the flap to the definitive recipient site
Partial necrosis of the flap occurred in three patients, and total necrosis was observed in only one patient (the patient with diabetes mellitus).
No differences in age, BMI, smoking history, flap dimensions, or arc of rotation were found to be statistically significant between patients with and without postoperative flap necrosis. Linear regression analysis did not show significant associations of BMI, flap size, and rotation angle with the saturation ratio.
No significant difference was found in before-transposition flap saturation between patients with and without necrosis. However, differences in after transposition SpO2 (61.6% vs. 89.6%, P = 0.025), ΔSpO2 (28.8% vs. 3.1%, P = 0.021) and the mean saturation ratio (32.3% vs 3.5%, P = 0.027) were found to be significant between patients with and without postoperative flap necrosis.
Despite the significant difference in the mean saturation ratio between the necrotic and non-necrotic flaps, no significant correlation was found between the saturation ratio and the percentage of necrotic area.
DISCUSSION
Perforator flap reconstruction is a transposition of skin islands, based on tiny arterial vessels usually smaller than 2 mm. Flap movement affects the perforator pedicle, but its effects may be detectable only after some hours after the operation. Prompt detection of a vascular issue in pedicled perforator flaps may help the surgeon to reduce the complication rate. Thus, surgeons have consistently pointed out the need for technical support to early predict possible vascular complications.
In 1975, Creech and Miller outlined the criteria they considered essential for an “ideal” free flap monitoring device [13]. These included safety to the patient and flap, applicability to all flap types, rapid response to blood flow changes, accuracy, affordability, and ease of interpretation [14]. Many techniques have been proposed, such as an implantable Doppler ultrasound probe, pulse oximetry, laser Doppler flowmetry, color Duplex sonography, NIRS, and tissue pH monitoring with microdialysis [15]. Although all these devices have improved flap salvage rates, none has proved to be superior to others.
NIRS is a tool for noninvasive measurement of tissue components. It is operator-independent and easy to manage, even by non-experts. It is important to avoid unstable probe contact and external light irradiation that can cause impossible or incorrect measurements. Regarding free flaps, NIRS has been reported to be a reliable technique to accurately detect decreases in flap perfusion (sensitivity, 99.1%; specificity, 99.9%) [14,16]. As reported in the literature, NIRS seems to detect vascular compromise of free flaps prior to clinical or Doppler monitoring [17,18].
Ozturk et al. [19] demonstrated no significant correlation between SpO2 and blood pressure, supplemental O2, flap type, perforator number, or vessel caliber. There is also no universally accepted consensus regarding a risk threshold during free-flap monitoring by NIRS, but the most widely supported criteria, proposed by Keller, indicate that a flap SpO2 lower than 30% or a drop rate in SpO2 equal to or greater than 20% per hour sustained for more than 30 minutes is predictive for vascular complications [20]. In our experiences with pedicled perforator flaps, an intraoperative drop rate in SpO2 ratio equal to or greater than 15%–20% was significantly associated with partial or total flap necrosis. Thus, intraoperative NIRS seems to be a useful tool to predict vascular issues in the very first minutes after flap inset, when it is otherwise clinically silent. However, it should be kept in mind that the NIRS probes commercialized for flap monitoring have a measurement depth within 10 mm [21].
We did not find significant correlations between the saturation ratio and the percentage of necrotic area. This finding may be related to the restricted number of analyzed patients, and further studies are needed to confirm or refute our results. Finally, we did not find significant associations of BMI, flap size, and rotation angle with the saturation ratio. These data seem to suggest that pedicled perforator flaps are equally safe in different patients and defects.
The average before-transposition SpO2 was overall good, ranging from 85% to 95%. Therefore, these perforator vessels were consistently sufficient to perfuse the entire skin paddle. In our case series, necrosis was most likely related to an insufficient length of the dissected pedicles to tolerate the torsion or minimal, but significant kinking. The ideal vessels should be larger than 0.5 mm in caliber and pulsatile, and the pedicle length should be at least 4 cm to reduce the risk of torsion.
An intraoperative drop rate in SpO2 ratio equal or greater than 15%–20% was predictive for vascular complications in pedicled perforator flaps. Conversely, flap size and rotation angle did not seem to be correlated with the risk of flap necrosis. Prompt detection of a vascular issue in pedicled perforator flaps could help the surgeon to reduce the complication rate; consequently, an immediate exploration of pedicle vessels is recommended to exclude the presence of kinking, twisting, or tension.
This is the first study regarding the immediate effect of flap transposition on vascularization, as measured using NIRS monitoring. The main limitations of our study are the limited population and the single-center design. Further studies are needed to analyze the efficacy of changes in the intraoperative reconstruction plan following immediate NIRS measurements.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
An official institutional review board (IRB) waiver of ethical approval was obtained from the IRB of Ospedale San Raffaele (exemption No. CE-11/int/2017) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: A Marchesi. Data curation: F Amendola. Formal analysis: F Amendola. Methodology: S Marcelli. Project administration: L Vaienti. Visualization: P Garieri. Writing - original draft: A Marchesi, F Amendola. Writing - review & editing: A Marchesi, F Amendola.