Direct-to-implant breast reconstruction following nipple-sparing mastectomy: predictive factors of adverse surgical outcomes in Asian patients
Article information
Abstract
Background
Direct-to-implant (DTI) breast reconstruction after nipple-sparing mastectomy (NSM) with the use of acellular dermal matrix (ADM) provides reliable outcomes; however, the use of ADM is associated with a higher risk of complications. We analyzed our experiences of post-NSM DTI without ADM and identified the predictive factors of adverse surgical outcomes.
Methods
Patients who underwent NSM and immediate DTI or two-stage tissue expander (TE) breast reconstruction from 2009 to 2020 were enrolled. Predictors of adverse endpoints were analyzed.
Results
There were 100 DTI and 29 TE reconstructions. The TE group had a higher rate of postmastectomy radiotherapy (31% vs. 11%; P=0.009), larger specimens (317.37±176.42 g vs. 272.08±126.33 g; P=0.047), larger implants (360.84±85.19 g vs. 298.83±81.13 g; P=0.004) and a higher implant/TE exposure ratio (10.3% vs. 1%; P=0.035). In DTI reconstruction, age over 50 years (odds ratio [OR], 5.43; 95% confidence interval [CI], 1.50–19.74; P=0.010) and a larger mastectomy weight (OR, 1.65; 95% CI, 1.08–2.51; P=0.021) were associated with a higher risk of acute complications. Intraoperative radiotherapy for the nipple-areolar complex increased the risk of acute complications (OR, 4.05; 95% CI, 1.07–15.27; P=0.039) and the likelihood of revision surgery (OR, 5.57; 95% CI, 1.25–24.93; P=0.025).
Conclusions
Immediate DTI breast reconstruction following NSM is feasible in Asian patients with smaller breasts.
INTRODUCTION
Nipple-sparing mastectomy (NSM) has gained considerable popularity over the past decades. The oncological results of NSM are comparable to those of skin-sparing mastectomy (SSM) in selected cases in terms of local recurrence and distant metastasis [1]. NSM is technically more challenging, with a higher complication rate than other approaches, given the additional risk posed by poor perfusion of the nipple-areolar complex (NAC) after mastectomy [2]. As a result, breast reconstruction following NSM can be more challenging and have a higher complication rate, especially in obese patients or in those with larger breasts [3]. Nevertheless, preservation of the NAC in mastectomy with immediate breast reconstruction, either implant-based or autologous, generally results in enhanced aesthetic results and, consequently, much better quality of life [3,4]. This procedure is now the standard of care when patients are eligible for it.
With the application of acellular dermal matrix (ADM), implant-based breast reconstruction has primarily shifted to direct-to-implant (DTI) procedures [5,6], especially when NSM is performed. Immediate implant-based breast reconstruction following NSM—either DTI or two-stage reconstruction with tissue expander (TE) implantation first—has been reported to have excellent aesthetic and safe oncological outcomes [4,7]. Nevertheless, whether DTI or two-stage reconstruction is superior remains inconclusive, with conflicting reports [8,9]. DTI reconstruction has been performed at our institution since the early 2000s, when ADM was not used in breast reconstruction procedures [10]. We have regularly performed DTI reconstruction after both SSM and NSM, with satisfying results in Asian patients, whose breasts are smaller than those of Western women [10]. Most of the studies discussing DTI reconstruction after NSM state that the use of ADM is required, and many of the NSMs reported in the literature were prophylactic mastectomies. Reports of clinical experiences of DTI reconstruction with NSM after breast cancer surgery without the use of ADM would be important, but are lacking.
The use of ADM in DTI breast reconstruction has been proven to lead to better overall outcomes and fewer complications than two-stage breast reconstruction. ADM provides adequate implant coverage in the inferior pole with improved aesthetic outcomes, better control of the inframammary fold (IMF), and less capsular contracture [11,12]. However, the use of ADM is also associated with a high number of complications, such as seroma, infection, and skin necrosis [13]. Our clinical experience of not using ADM seems to provide another option for DTI when performing breast reconstruction in Asian patients. The main purpose of this study was to present a comprehensive review of our experience and to provide a useful reference for patient selection for DTI without ADM.
METHODS
After receiving approval from the institutional review board committee (IRB Nos. 202000235B0 and 202100103B0), this retrospective study recruited patients who received implantbased immediate breast reconstruction from the senior author ( JJH) following NSM from October 2009 to April 2020. Patients were excluded if they had received NSM for risk-reducing prophylactic mastectomy or mastectomy to remove disseminated foreign bodies, including injected silicone and reacted granuloma, or if they were initially planned to receive autologous breast reconstruction after NSM and TE insertion. The minimal follow-up time was 3 months.
Patients were grouped into a DTI reconstruction group and a two-stage reconstruction with TE implantation first group (hereafter referred to as the TE group). The demographic and clinical data obtained included age, body mass index, smoking history, medical history (e.g., diabetes mellitus, hypertension, previous breast cancer and surgery), cancer-related characteristics (e.g., side, location, distance between the tumor and nipple, biological type of breast cancer, and TNM stage), and perioperative treatment information, including incision placement for mastectomy, axillary lymph node dissection, intraoperative radiotherapy (IORT) to the NAC, neoadjuvant and adjuvant chemotherapy, and postmastectomy radiotherapy (PMRT). Acute complications included poor wound healing, mastectomy skin flap partial necrosis, nipple necrosis, seroma, hematoma, and infection, while late complications included late wound dehiscence, infection, capsular contracture, implant/expander rupture or exposure, and implant/expander loss. We also recorded whether aesthetic revision surgery was performed for purposes including scar release, capsular contracture release, and fat grafting. Local recurrence and distant metastasis were reviewed.
Surgical technique
After the breast surgeons finished the mastectomy, the reconstruction team took over. The perfusion of the NAC and mastectomy skin flap was assessed to ensure a well-perfused breast skin envelope before implant or TE insertion; this was achieved using an intravenous injection of indocyanine green, which was visualized using a SPY device (Stryker Corp./Novadaq Technologies, Kalamazoo, MI, USA) if required (Fig. 1). If the perfusion of the skin envelope or NAC was compromised, the DTI was converted to TE insertion.
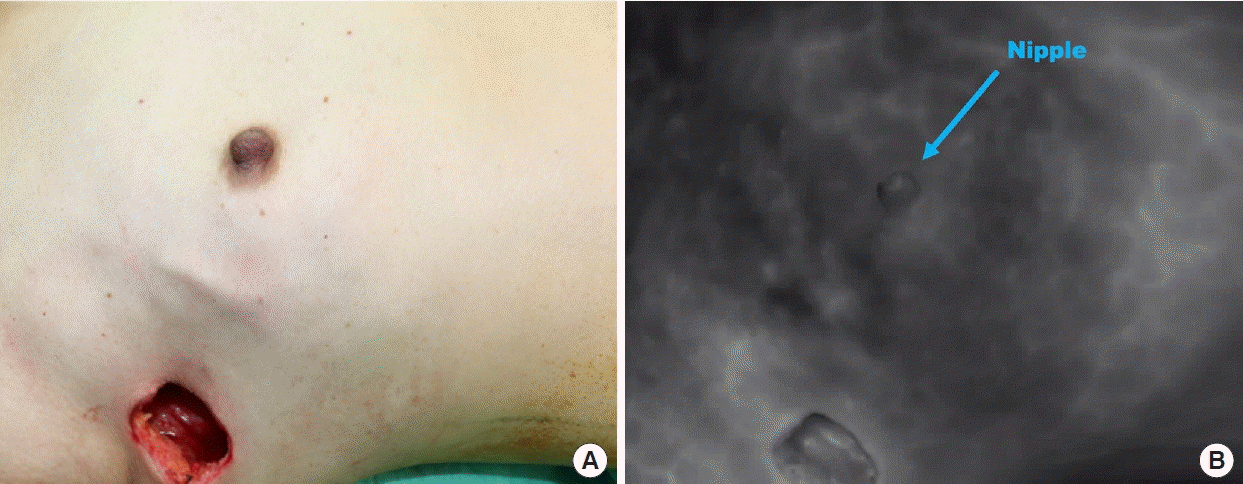
Intraoperative photography grossly showing good perfusion of the preserved skin flap and the nipple in a nipple-sparing mastectomy before implant reconstruction (A), which was further confirmed by intravenous indocyanine green injection and visualized using a SPY machine (B).
If the NAC and skin envelope were both confirmed to be well perfused, the subpectoral plane was created with careful dissection and ligation of the underlying perforators nourishing the pectoralis major (PM) muscle. The insertion of the PM muscle below the fourth intercostal level was divided. The inferior mammary fold was reinforced using 3-0 polydioxanone sutures. If the mastectomy extended beyond the anterior axillary line, two stitches were performed to recreate the lateral fold of the breast on the anterior axillary line. A mammary sizer was first inserted to ensure adequate pocket creation and to select the implant size. After that, the pocket was irrigated with a large amount of saline, and secure hemostasis was achieved. A suction drain was inserted. Before finalizing the surgery, two stitches to anchor the inferior margin of the PM muscle to the spared skin flap were placed using Vicryl sutures to prevent muscle retraction without coverage of the implant. This helped ensure that the PM muscle would stay in the planned position and prevent the implant from sliding into the prepectoral space before complete capsule formation. The breast implant was then placed. Before wound closure, to provide better control of the lateral pocket for the breast implant, two stitches were placed to anchor the dermis of the anterior axillary line to the corresponding underlying periosteum. The stitches helped prevent lateral displacement of the implants. After wound healing, the lateral border of the reconstructed breast would then be formed. The wound was closed primarily. Patients received parenteral prophylactic antibiotics postoperatively for 3 days before being discharged.
Statistical analysis
The statistical analysis was conducted using SPSS version 23.0 (IBM Corp., Armonk, NY, USA). The chi-square or Fisher exact test was used for categorical data, while the Student t test or Mann-Whitney U test was utilized for continuous data. Factors contributing to surgical outcomes were analyzed with univariable logistic regression, and factors with a P-value < 0.2 in the univariable analysis were selected for the multivariable analysis. Backward selection was used to obtain the final models for four outcomes, in which variables with P < 0.1 were selected. A two-sided P-value < 0.05 was considered to indicate statistical significance.
RESULTS
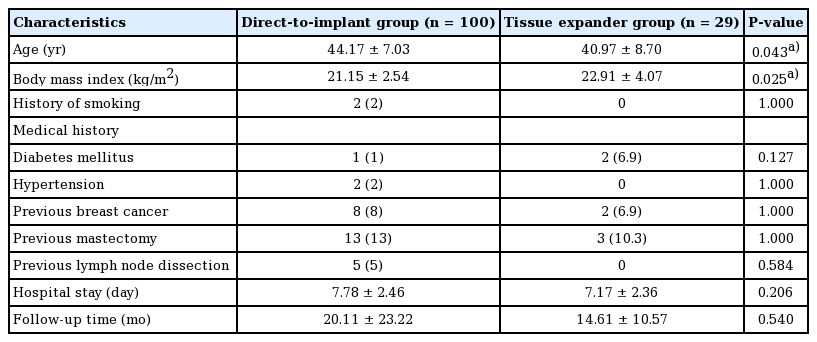
A total of 129 breasts in 123 patients undergoing immediate breast reconstruction following NSM were enrolled, including 100 and 29 cases in the DTI and TE groups, respectively. Table 1 lists their demographic characteristics. The patients in the TE group were younger (40.97 ± 8.70 years vs. 44.17 ± 7.03 years; P = 0.043) and had a higher average body mass index (22.91 ± 4.07 kg/m2 vs. 21.15 ± 2.54 kg/m2; P = 0.025) than those in the DTI group (Table 1). The breast cancer characteristics and treatments are shown in Table 2; no significance between-group differences were found in the biological type of the cancer, the cancer stage, nodal surgery, neoadjuvant/adjuvant chemotherapy, or hormone therapy. More patients received PMRT in the TE group than in the DTI group (31% and 11%, respectively; P = 0.009).
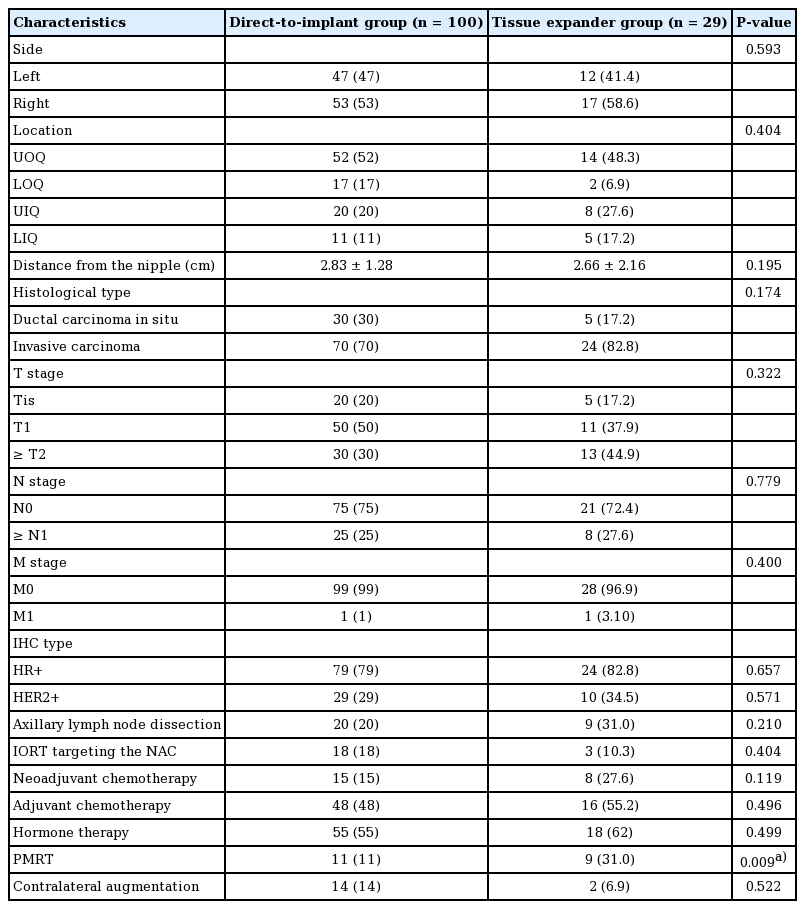
Tumor characteristics and associated treatment between the direct-to-implant and tissue expander groups
The postoperative outcomes are summarized in Table 3. The TE group had larger mastectomy specimens (317.37 ± 176.42 g and 272.08 ± 126.33 g, respectively; P = 0.047) and larger implants (360.84 ± 85.19 g and 298.83 ± 81.13 g, respectively; P = 0.004). Acute and late complications were similar between the groups, except that late implant/expander exposure was significantly more frequent in the TE group (10.3%) than in the DTI group (1%) (P = 0.035). Six of the 29 (20.7%) patients in the TE groups were converted to free flap reconstruction due to the patient’s preference after TE insertion.
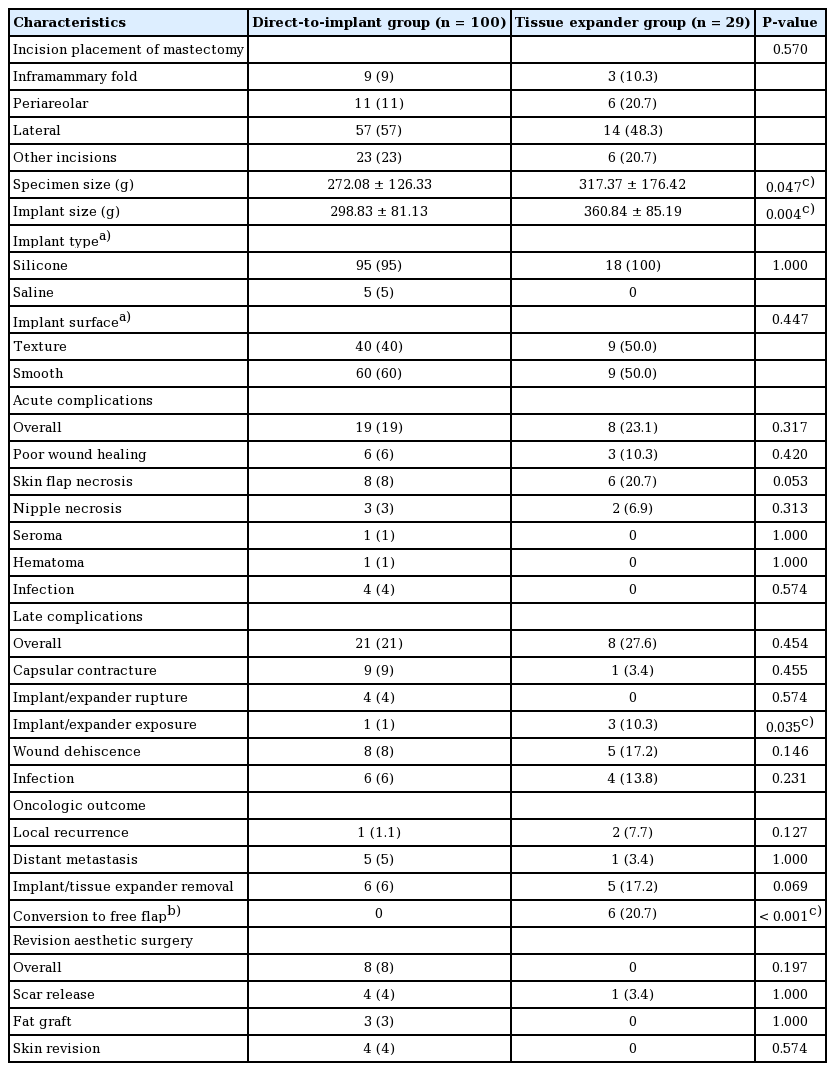
Intraoperative data and postoperative outcomes between the direct-to-implant and tissue expander groups
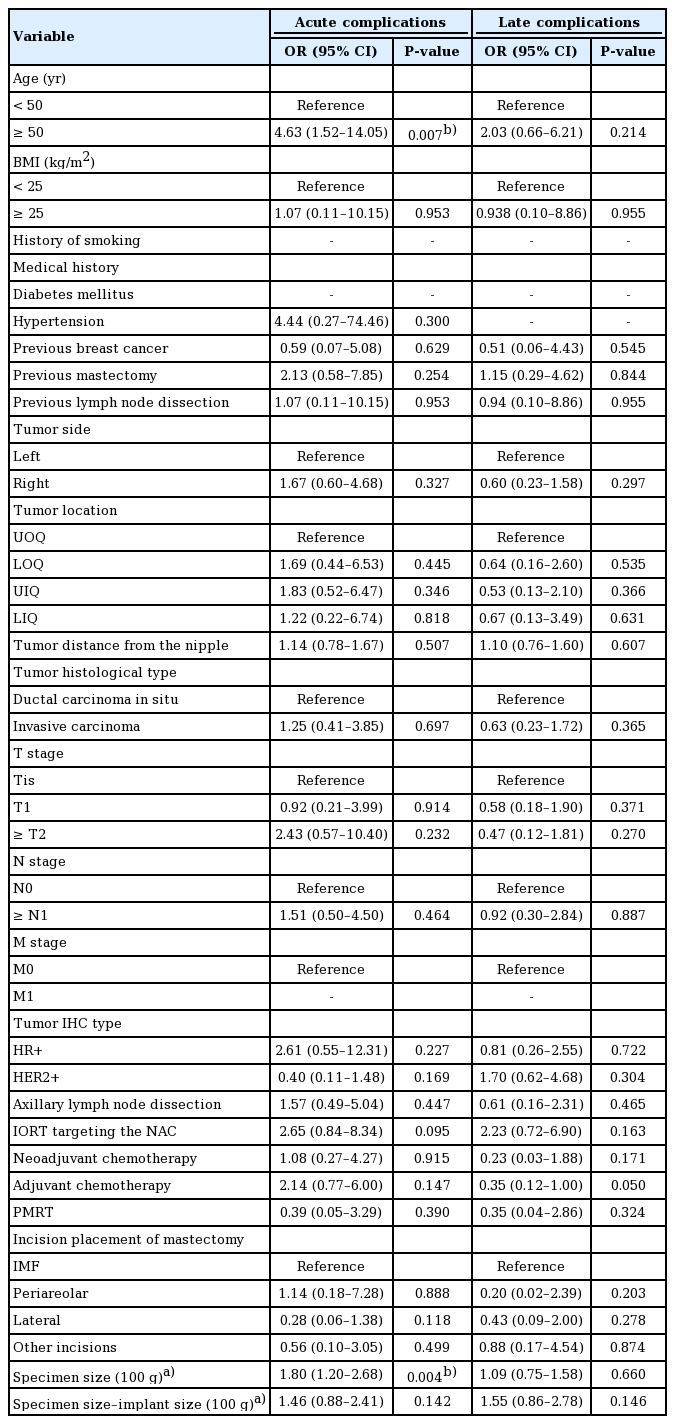
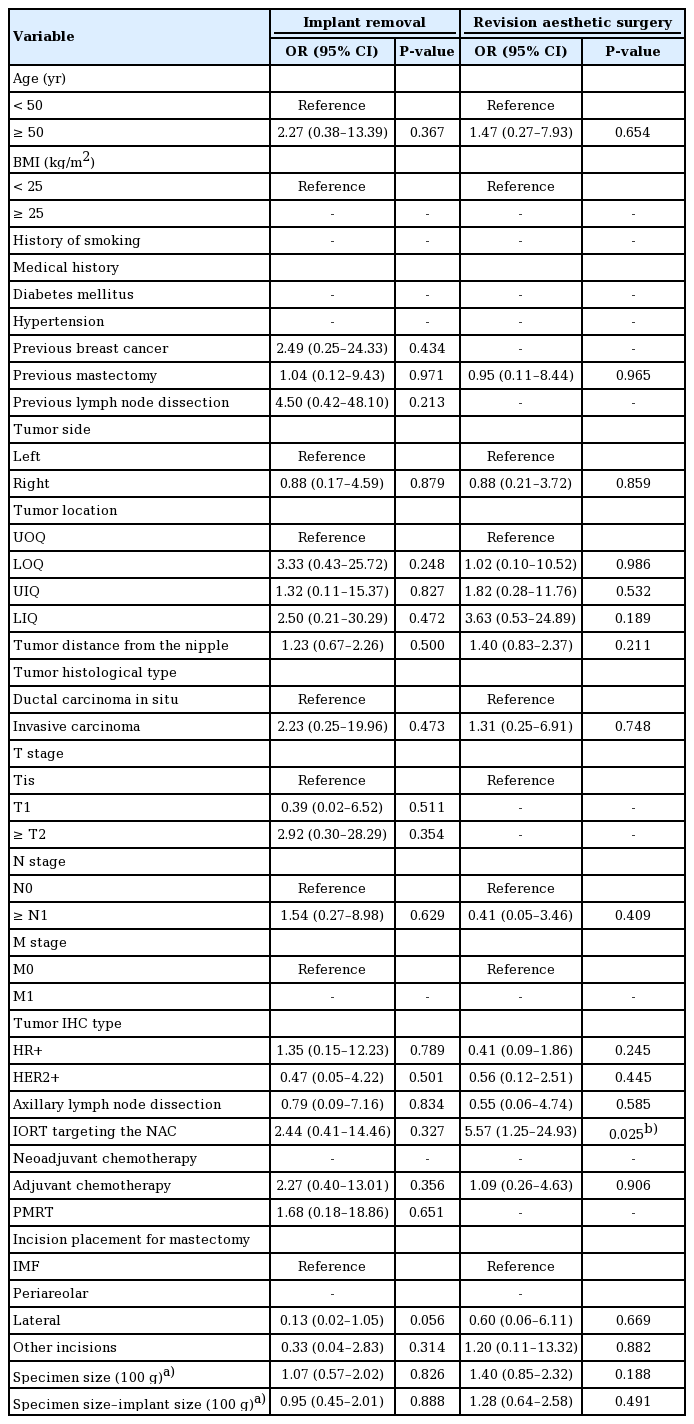
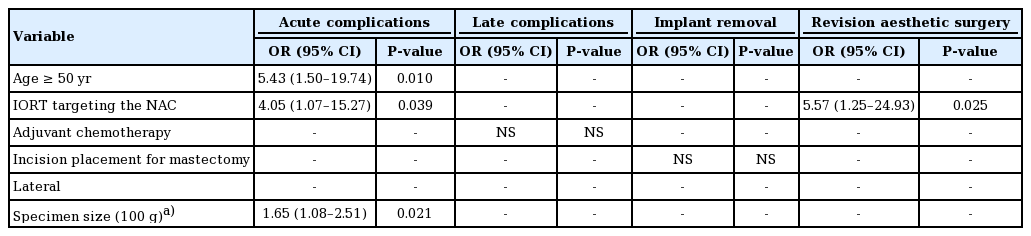
To identify factors contributing to acute and late complications, univariate logistic regression was conducted for overall complications as a whole and for implant removal and aesthetic revision surgery in particular, as shown in Tables 4 and 5. Age older than 50 years (odds ratio [OR], 4.63; 95% confidence interval [CI], 1.52–14.05; P = 0.007) and a larger mastectomy weight (per 100 g) (OR, 1.80; 95% CI, 1.20–2.68; P = 0.004) were associated with a higher risk of overall acute complications. IORT targeting the NAC (OR, 5.57; 95% CI, 1.25–24.93; P = 0.025) increased the risk of revision surgery. Multivariable logistic regression was further conducted for variables with P < 0.1 in the univariate model. Consistent with the univariate logistic regression, age greater than 50 years (OR, 5.43; 95% CI, 1.50–19.74; P = 0.010) and a larger mastectomy weight (per 100 g) (OR, 1.65; 95% CI, 1.08–2.51; P = 0.021) were associated with a higher risk of acute complications. IORT targeting the NAC contributed to both a higher risk of acute complications (OR, 4.05; 95% CI, 1.07–15.27; P = 0.039) and to a higher likelihood of requiring aesthetic revision surgery (OR, 5.57; 95% CI, 1.25–24.93; P = 0.025). Although advanced age, mastectomy weight, and IORT targeting the NAC were found to be associated with a higher overall complication rate and a higher likelihood of requiring revision surgery, neither factor was associated with any specific complication when the complications were analyzed individually (Table 6).
DISCUSSION
Our findings supported the hypothesis that, with proper patient selection, DTI reconstruction can be successfully performed after NSM for breast cancer treatment in Asian patients with a low body mass index and small to moderate-sized breasts, even without the use of ADM (Figs. 2, 3).
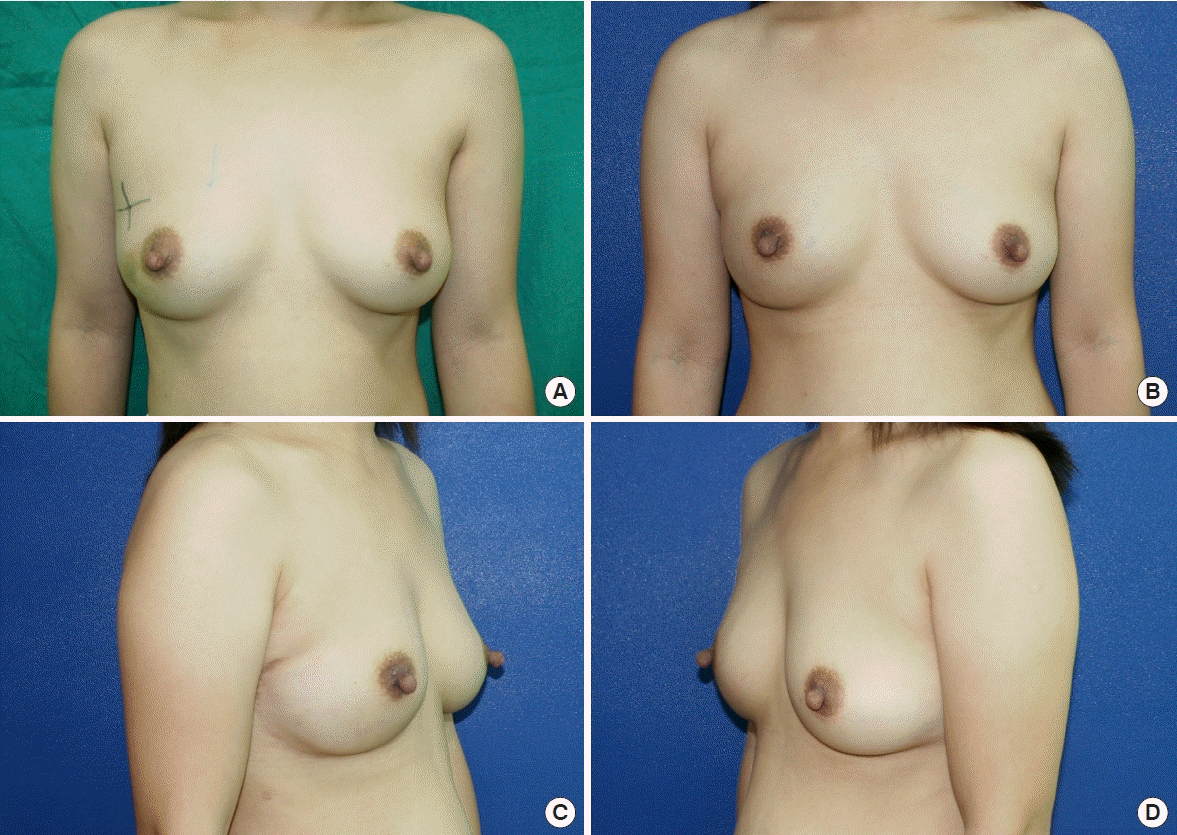
Photographs of a 39-year-old patient who underwent right-side direct-to-implant breast reconstruction following nipple-sparing mastectomy for ductal carcinoma in situ without the use of acellular dermal matrix. (A) Preoperative anterior view. Postoperative anterior view (B), right lateral view (C), and left lateral view (D). Postoperative photos were taken at the 5-month follow-up.
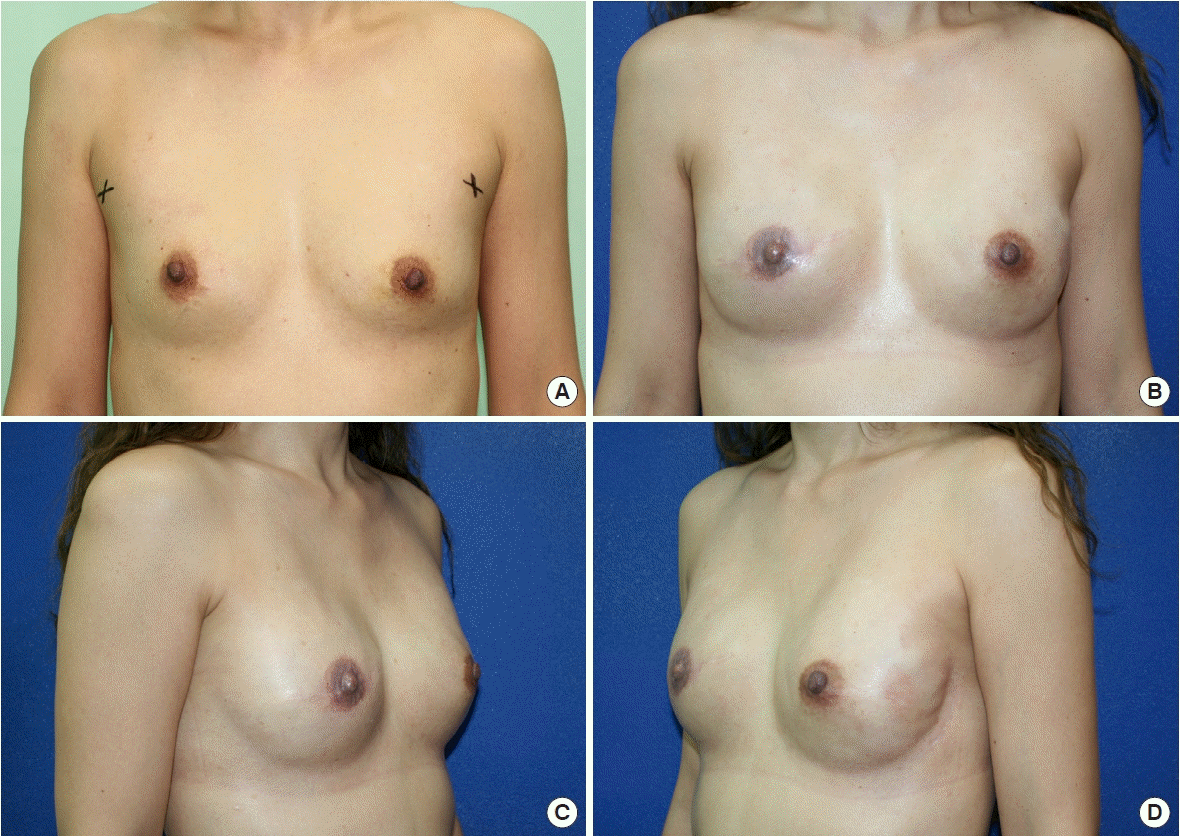
A 47-year-old female patient with bilateral breast cancer, staged T1 miN0 on the right side and atypical ductal hyperplasia on the left side. She received double mastectomy, with skin-sparing mastectomy on the right side and nipple-sparing mastectomy on the left side. Simultaneous bilateral direct-to-implant reconstruction was performed immediately. (A) Preoperative anterior view. Postoperative anterior (B), right lateral (C), and left lateral (D) views after right-side nipple and areolar reconstruction at the 12-month follow-up.
Immediate DTI breast reconstruction was first introduced in the early 2000s and popularized after ADM was applied for implant-based reconstruction [10,14]. However, conflicting results have been reported, and the preference for DTI reconstruction over two-stage implant-based reconstruction seems to be highly reliant on the use of ADM [8,11,15-20]. As presented herein, we found that the frequency of implant/TE exposure was significantly lower in the DTI group (1%) than in the TE group (12.3%, P = 0.032) (Table 3). However, selection bias may have affected these findings, since two-stage reconstruction has long been suggested if PMRT is required, and we tend to perform two-stage reconstruction if PMRT is required or if the perfusion of the mastectomy skin flap is compromised.
Implant loss was observed in both groups for different reasons. In the DTI group, six cases of late implant loss were encountered, with two due to capsular contracture and removal at the patient’s request, three due to infection, and one due to delayed repeated wound dehiscence after radiotherapy. In the TE group, TE/implant loss occurred in five patients: one who developed capsular contracture and requested removal, three who experienced repeated wound disruption with TE/implant exposure, and one who developed breast cellulitis. Six patients decided to convert from implant-based reconstruction to autologous reconstruction.
Despite the advantages of DTI reconstruction, several factors increased the risk of complications, including age over 50 years, a larger mastectomy specimen, and delivery of IORT targeting the NAC. The size of the mastectomy has been reported as a factor contributing to adverse outcomes in DTI breast reconstruction by various authors [17,18,21]. In our study, the size of the mastectomy specimen was also found to be a significant contributing risk factor for acute complications of DTI reconstruction (OR, 1.65; 95% CI, 1.08–2.51; P = 0.021). Interestingly, while mastectomy size negatively impacted the surgical outcomes, the same finding did not apply to the size of the breast implants (Table 4). Similar findings were presented by Negenborn et al. [21], who showed that the complication rate was associated with the size of the mastectomy specimen, but not the size of the inserted breast implant. A possible reason for this is that the implants used for reconstruction tend to be smaller than the mastectomy specimens, and therefore were not significantly different from one another.
In addition, age greater than 50 years contributed to a higher acute complication rate (OR, 5.43; 95% CI, 1.50–19.74; P = 0.010), which has also been reported as a risk factor by Hunsicker et al. [22]. Larger breasts and the breasts of women of more advanced age are associated with ptosis, and the collateral circulation from the surrounding normal tissue to the breast envelope of ptotic breasts is relatively more restricted, resulting in more frequent skin necrosis and a larger dead space for hematoma and seroma accumulation.
IORT targeting the NAC during NSM has been reported to improve local cancer control and radiation safety [23]. Our approach includes an INTRABEAM IORT device (Zeiss Meditech, Jena, Germany) with a single dose of 6–12 Gy to irradiate the NAC, followed by immediate breast reconstruction. IORT targeting the NAC was associated with a higher risk of acute complications in our small series (OR, 4.05; 95% CI, 1.07–15.27; P = 0.039) and a higher frequency of aesthetic revision surgery (OR, 5.57; 95% CI, 1.25–24.93; P = 0.025). There was a higher likelihood of requiring revision surgery, including nipple revision, nipple reconstruction, and scar revision on the incision site, since IORT targeting the NAC obstructs the collateral circulation through the NAC to the incision site. The risks of aesthetic complications have rarely been discussed in the literature. Both our univariate and multivariate logistic regression analyses identified IORT as a contributing factor to acute complications and the need for secondary aesthetic touchup.
Unlike previous publications [24], in our series, incision placement had no significant relationship with adverse surgical outcomes. In previous studies regarding various incision patterns, making the incision through the IMF yielded more favorable outcomes [25]. We have relatively little experience with the IMF approach (9%), whereas a lateral incision (57%) was the most widely used approach due to aesthetic considerations and the possible need for future axillary nodal surgery.
The application of ADM in implant-based reconstruction has increased in the last 10 years [11,26]. However, the use of ADM increases medical costs, and ADM implantation also inevitably results in a higher risk of some complications, such as infection or seroma formation [13,22,26,27]. To balance the costs, reduce the complications, and enhance the aesthetic results, it is important to select candidates who would benefit more from ADM or no ADM in DTI reconstruction. The application of ADM has been recommended in DTI breast reconstruction and it is considered helpful when larger breast implants are required [11,26,28,29]. In the current study, we successfully performed DTI reconstruction after NSM without ADM. Although the implants did not have support in the inferior pole, we did not experience bottom-up or implant migration from the subpectoral to the prepectoral plane. These favorable outcomes were believed to be related to the surgical techniques used to fix the PM muscle to the skin flap and the smaller implants required in our population. Breast size represents a significant difference between Asian and Western populations; consequently, the breast implants used for breast reconstruction in our series were much smaller than those reported in most previous studies. Ninety-three of the 100 mastectomies used implants that were smaller than 500 g, and the breast implants used in our patient cohort were generally small (298.83 ± 81.13 mL on average). Our results suggest that post-NSM DTI reconstruction without ADM application is feasible, with reasonable complication rates and satisfying aesthetic results, when the reconstructed breasts are small and non-ptotic.
Despite the satisfactory results, there are a few limitations of our study. First, a control group of patients who underwent DTI reconstructions with ADM was lacking. However, this fact reflects our early start and consistent performance of DTI without the use of ADM. Second, the outcomes demonstrated in our study were all on the surgical side; patient-reported outcomes will be obtained later to confirm these results. Third, the number of patients remains small, and a larger-scale study needs to be carried out in the future. Fourth, the follow-up duration varied, with the shortest period being only 3 months. A study with a longer follow-up time will be continued to evaluate long-term outcomes. Finally, the choice between DTI and TE was not randomly controlled, and we tended to perform DTI if possible. Only patients who had poorly perfused skin envelopes, inadequate skin envelopes, or were known to need PMRT received TE insertion after mastectomy. This might have contributed to their higher frequency of complications, particularly skin flap necrosis and wound healing problems. Selection bias might have contributed to the higher complication rate in the TE group.
Although more complications tended to occur in patients with larger breasts and those over 50 years old, DTI breast reconstruction after NSM remains a feasible and safe procedure with reasonable overall acute and late complications in highly selected Asian patients.
Notes
Conflict of interest
Jung-Ju Huang is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Ethical approval
The study was approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB Nos. 202000-235B0 and 202100103B0) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: WL Kuo, SC Chen, JJ Huang. Data curation: WL Kuo, JJ Huang. Formal analysis: CL Su, JR Yang, JJ Huang. Methodology: WL Kuo, SC Chen, DC Cheong, JJ Huang. Project administration: WL Kuo, SC Chen, JJ Huang. Visualization: CL Su, JR Yang, WL Kuo, DC Cheong, JJ Huang. Writing - original draft: CL Su, JR Yang, JJ Huang. Writing - review & editing: WL Kuo, SC Chen, DC Cheong, JJ Huang.