Volumetric change of the latissimus dorsi muscle after postoperative chemotherapy and radiotherapy in immediate breast reconstruction with an extended latissimus dorsi musculocutaneous flap: final results from serial studies
Article information
Abstract
Background
Breast reconstruction using an extended latissimus dorsi (eLD) flap can supplement more volume than reconstruction using various local flaps after partial mastectomy, and it is a valuable surgical method since the reconstruction area is not limited. However, when performing reconstruction, the surgeon should consider latissimus dorsi (LD) volume reduction due to postoperative chemotherapy (POCTx) and postoperative radiotherapy (PORTx). To evaluate the effect of POCTx and PORTx on LD volume reduction, the effects of each therapy—both separately and jointly—need to be demonstrated. The present study quantified LD volume reduction in patients who underwent POCTx and PORTx after receiving breast-conserving surgery (BCS) with an eLD flap.
Methods
This study included 48 patients who received immediate breast reconstruction using an eLD flap from January 2013 to March 2017, had chest computed tomography (CT) 7–10 days after surgery and 10–14 months after radiotherapy completion, and were observed for more than 3 years postoperatively. One surgeon performed the breast reconstruction procedures, and measurements of breast volume were obtained from axial CT views, using a picture archiving and communication system. A P-value <0.05 was the threshold for statistical significance.
Results
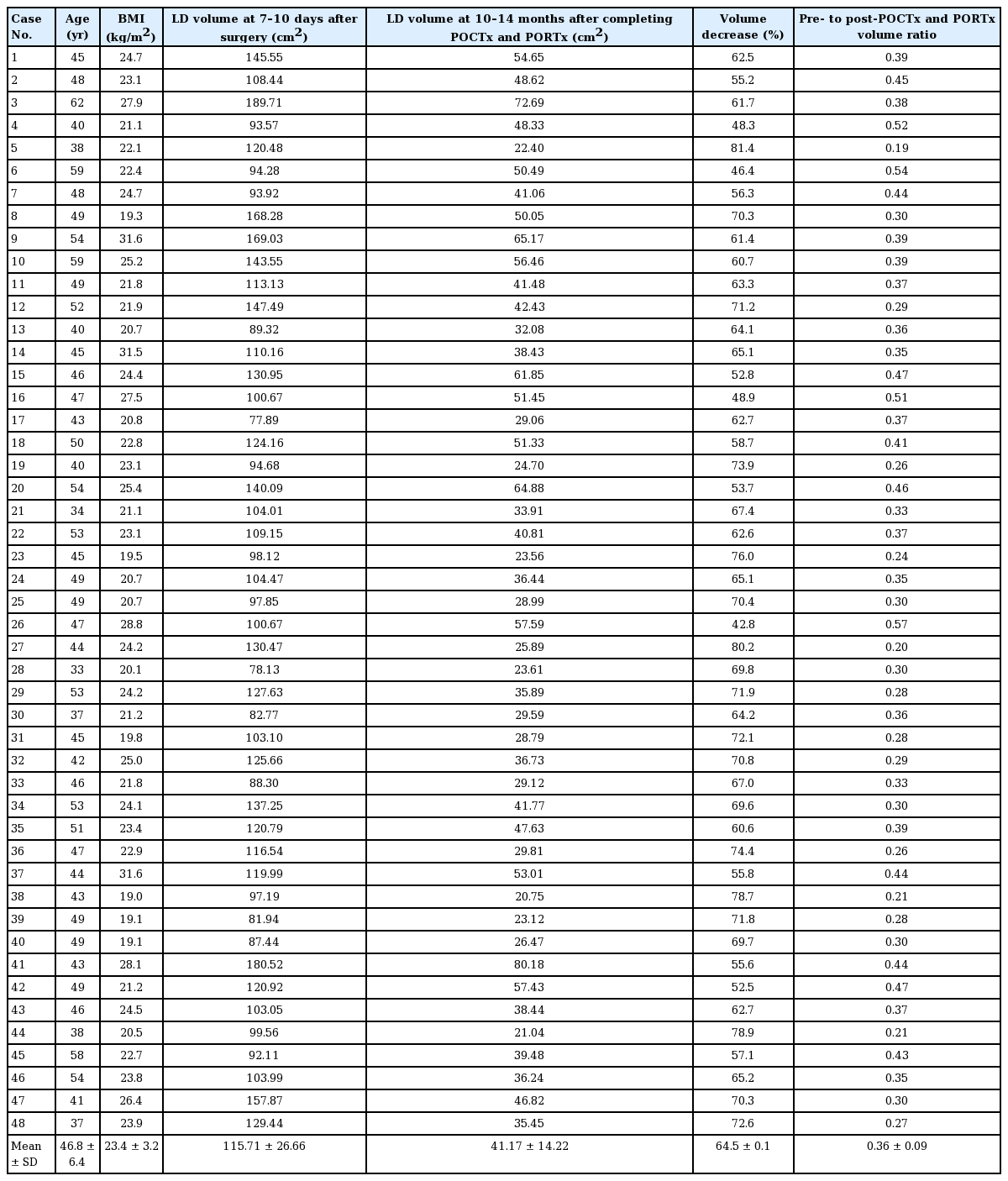
The average volume reduction of LD at 10–14 months after completing POCTx and PORTx was 64.5% (range, 42.8%–81.4%) in comparison to the volume measured 7–10 days after surgery. This change was statistically significant (P<0.05).
Conclusions
Based on the findings of this study, when harvesting an eLD flap, surgeons should anticipate an average LD volume reduction of 64.5% if chemotherapy and radiotherapy are scheduled after BCS with an eLD flap.
INTRODUCTION
The extended latissimus dorsi (eLD) flap is a method used in breast-conserving surgery (BCS) that independently allows breast reconstruction without implants even after total mastectomy in patients with small breasts [1-3]. Since the symmetry of the reconstructed breast with the contralateral breast is an essential aspect of breast reconstruction, volume reduction due to atrophy of the latissimus dorsi (LD) needs to be considered when performing breast reconstruction using an eLD flap. Some factors may influence the atrophy of the LD after breast reconstruction with an eLD flap. First, it can be predicted that a large postoperative reduction in breast volume will result from resecting the motor nerve to the LD to prevent synkinetic movement of the LD [4,5]. A second factor to consider is LD volume reduction resulting from postoperative chemotherapy (POCTx) or postoperative radiotherapy (PORTx); in particular, fibrosis of the remnant breast tissue and flap after PORTx makes it difficult to predict the long-term outcomes of breast reconstruction [6].
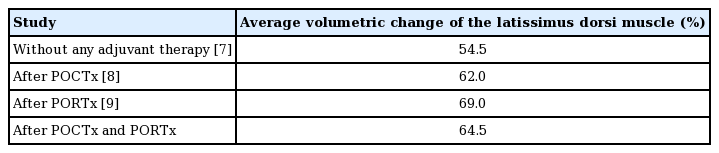
The authors have carried out multiple studies of volume reduction of the LD after breast reconstruction using the eLD flap, and demonstrated that the extent of LD atrophy differed between patients who received neither POCTx nor PORTx and those who received either POCTx or PORTx [7-9]. The average reduction of LD volume was 54.5% when receiving reconstruction with an eLD flap after total mastectomy without POCTx or PORTx, 62.0% when only POCTx was performed, and 69.0% when only PORTx was carried out. This study aimed to build upon the results of those previous studies by evaluating the amount of LD volume reduction in patients who underwent both POCTx and PORTx after receiving BCS with an eLD flap.
METHODS
Participants
Of the 147 patients who underwent BCS with an eLD flap from January 2013 to March 2017, the target subjects of this study included 48 patients who received chest computed tomography (CT) scans at 7–10 days postoperatively and at 10–14 months after PORTx completion, and were under observation for more than 3 years (mean, 58.6 months; range, 37–88 months). The average age of the patients was 46.8 years, and their average body mass index was 23.4 kg/m2. A single surgeon performed breast reconstruction and resected the motor nerve innervating the LD in all patients. PORTx was performed after the completion of POCTx, and a high-energy linear accelerator (Clinical IX; Varian, Palo Alto, CA, USA) was used to irradiate the entire breast on the lesion side. The general tangential fields technique was used to administer a total radiation dose of 50.4 Gy by conventional fractionation for a period of 5–6 weeks. An additional 10 Gy was then administered at the site of tumor resection.
Surgical technique
After performing partial mastectomy and axillary dissection, a thoracodorsal pedicle pathway was found through the axillary region. A skin paddle was designed with a width of 2–5 cm and a length of 10–15 cm. An eLD flap was harvested using the conventional technique. After complete division of the LD near its origin, a thoracodorsal pedicle was completely isolated, and motor nerve to the LD was then ligated and excised, with a length of 1 cm, in order to prevent synkinetic movement of the LD. While insetting the flap, the upper body was elevated 60° to a near-sitting position. The eLD flap was folded and secured, considering the pocket of the defect. When a portion of breast skin or the nipple-areolar complex (NAC) was removed, the skin paddle was positioned outwardly. In cases where the NAC was preserved, part of the de-epithelialized skin paddle was always positioned under the NAC region.
LD volume measurements
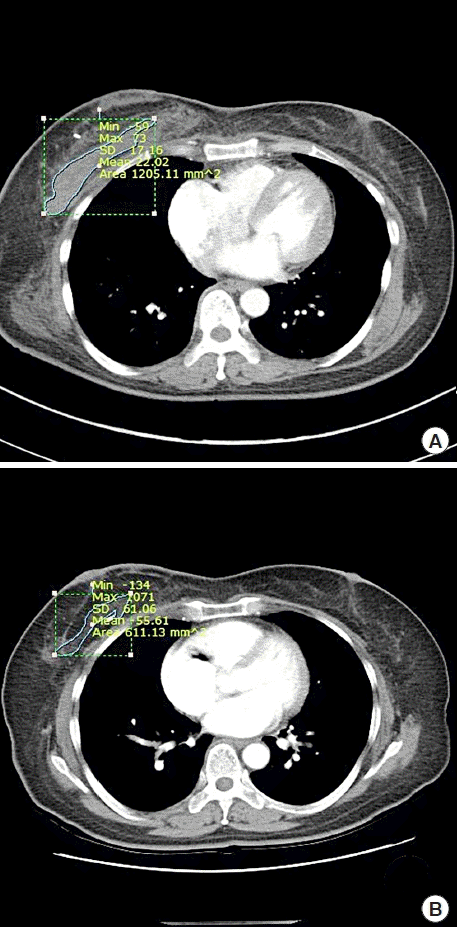
All patients underwent follow-up CT scans 7–10 days after surgery and 10–14 months after the completion of POCTx and PORTx. No contrast medium was used, and images were taken at a 3-mm or 5-mm slice thickness including the entire chest area. The breast reconstructive surgeon obtained measurements of the LD using the picture archiving and communications system from the axial view of chest CT scans (Fig. 1). The total LD volume was calculated by multiplying the total measured area by the thickness of the CT slices. The measurements were verified by an experienced professor from the radiology department who specialized in breast imaging to minimize individual differences in measurements. The volume measurements were statistically analyzed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA), with P < 0.05 as the threshold for statistical significance.
Statistical analysis
The Shapiro-Wilk test was conducted to test whether these values had a normal distribution, and the pre-therapy values (P = 0.005) and the post-POCTx and PORTx values (P = 0.058) yielded P-values smaller and larger than 0.05 respectively. Therefore, both parametric (paired t-test) and non-parametric (Wilcoxon signed-rank test and the sign test) methods were used. These statistical analyses were conducted in SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
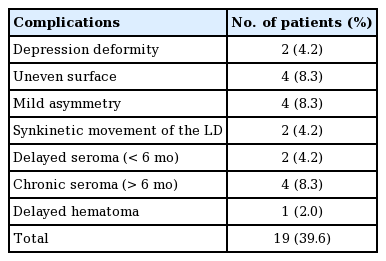
As measured on the chest CT scans of 48 patients taken 7–10 days after surgery, the LD volume ranged from 77.89 cm3 to 189.71 cm3, with an average of 115.71 cm3. The LD volume measured on chest CT scans taken 10–14 months after the completion of POCTx and PORTx ranged from 20.75 cm3 to 80.18 cm3, with an average of 41.17 cm3. The average volume reduction of the LD was 64.5 cm3 (range, 42.8–81.4 cm3), which was statistically significant (P < 0.05) (Table 1). In the follow-up period of at least 3 years, there were two cases of depression atrophy lesions (one in the lower inner quadrant and one in the lateral half), but the patients did not request retouching. The other patients, including four with an uneven surface remaining at the surgical site, were all satisfied with the outcomes (Fig. 2). Overcorrection was performed in all patients by ensuring that the volume of the flap exceeded the volume of resected tissue. In four cases, the contralateral breast became larger or ptotic due to weight gain, and in two cases, the patients complained about the inconvenience of synkinetic movement of the LD. Regarding donor site complications, there were four cases of chronic seroma lasting longer than 6 months, two cases of delayed seroma, and one case of delayed hematoma for which surgical resection was performed. No other complications, such as infections or recurrence, were reported (Table 2).
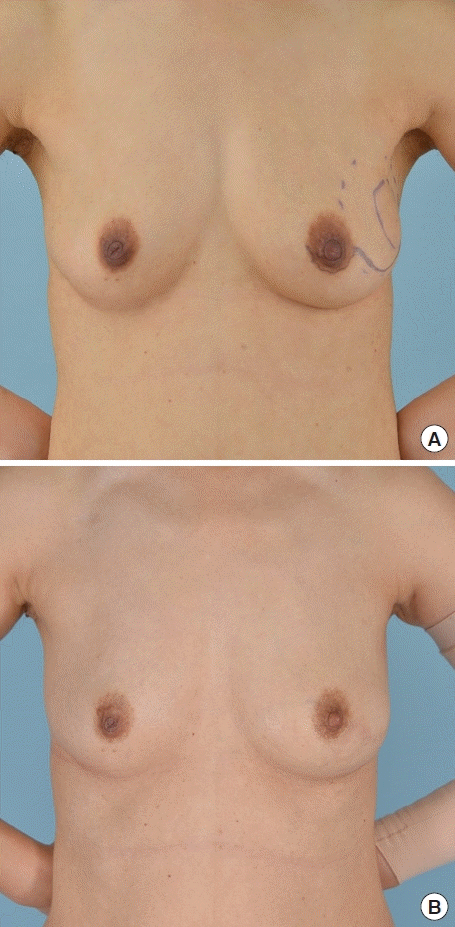
Preoperative and postoperative photography. The patient was 49 years old and had left breast cancer (T2N0M0). Her body mass index was 20.7 kg/m2. The excised volume was 87.6 g. Photographs were taken (A) preoperatively, and (B) at 3 years postoperatively.
DISCUSSION
The percentage of patients undergoing concurrent breast reconstruction after mastectomy in Korea has steadily increased from 19.4% in 2015 to 53.4% in 2018 [10]. In 2019, breast cancer made up 23.8% of all cancers in women, which is 4-fold higher than the proportion 20 years ago, and breast cancer has become the most common malignant tumor in women [11]. Since breast reconstruction can provide aesthetic and psychological benefits and improve quality of life, increasingly many breast cancer patients are undergoing breast reconstruction [12].
In BCS procedures using the eLD flap, the breast volume at the time of surgery is not maintained due to postoperative LD atrophy. Since POCTx and PORTx are often performed after breast reconstruction with an eLD flap, it is necessary for surgeons to understand the extent of LD volume reduction induced by those therapies. Therefore, in previous studies, we investigated the effects of POCTx and PORTx on LD volume reduction. The first study was conducted among patients who did not receive either POCTx or PORTx, and the second study was conducted among patients who received only POCTx. In both studies, implants were used simultaneously. The third study was conducted among patients who received only PORTx and underwent BCS using an eLD flap. However, important limitations of those studies were that different surgical methods were performed and that the timing of LD volume calculation was inconsistent. Nipple-sparing mastectomy or skin-sparing mastectomy should be performed in patients who are not planning to receive POCTx or PORTx. At the time when the procedures were carried out, it was conventional to use implants when performing breast reconstruction using an eLD flap. Therefore, it was necessary to make indirect comparisons of the serial results since a direct comparison was impossible due to differences in the presence or absence of implants. In addition, since the LD volume should be calculated after completing adjuvant therapy if a patient receives POCTx or PORTx, it was inevitable that the timing of the LD volume calculation was different in each study. However, this study nonetheless makes a meaningful contribution by confirming that patients’ breast shape minimally changed after a minimum of a 1-year follow-up following the completion of adjuvant POCTx or PORTx.
The authors evaluated the extent of LD atrophy in a previous study wherein the breast was reconstructed using an eLD flap with an implant after total mastectomy without the influence of POCTx and PORTx, and observed that the average LD atrophy was 54.5% at 6–8 months after reconstruction surgery [7]. We also found that when patients received only POCTx after breast reconstruction using the same method, the average extent of LD atrophy at 6–14 months after chemotherapy was 62.0% [8]. Our third study showed that when patients received breast reconstruction with an eLD flap as BCS and received only PORTx, the average extent of LD atrophy at 14–22 months was 69.0% (Table 3) [9]. These three earlier studies demonstrated differences in the extent of LD atrophy, providing a basis for evaluating the variance in the extent of LD flap atrophy in patients who received both POCTx and PORTx after reconstruction with an eLD flap.
In this study, the patients received BCS with an eLD flap and underwent PORTx, usually 1 month after POCTx was completed. The average LD volume reduction at 10–14 months after the completion of PORTx was 64.5%. Compared to our previous studies, this volume change was greater than that of patients receiving neither POCTx nor PORTx, similar to that of patients receiving only POCTx, and less than that of patients receiving PORTx alone. It is noteworthy that the greatest LD atrophy was seen in patients who only received PORTx, and it is our opinion that radiotherapy has a greater effect on fresh flaps by inducing fibrosis when it is performed around 1 month after surgery. POCTx provides time for the flap to stabilize postoperatively, as demonstrated by the fact that the patients who received PORTx after POCTx showed equivalent results to those observed in patients who received only POCTx. In patients who received neither POCTx nor PORTx, there was a small difference in the extent of LD flap atrophy at 18 months postoperatively compared to 6–8 months after surgery. However, a limitation of identifying the contour of the LD on CT using the picture archiving and communications system is that it is extremely difficult to conduct measurements after 2 years have passed since the operation. For this reason, the final interval for the CT scans was approximately 18 months postoperatively in this study, and it was difficult to recognize the precise LD contour on CT scans taken later than that. However, a clinical assessment of the morphology and size of the breast through patient interviews and observations showed that minimal to no volume reduction took place more than 1 year after the completion of radiotherapy, suggesting that the results of our study are meaningful.
When comparing cases using only an eLD flap with cases where an implant is also used, it should be kept in mind that the volume of the implant might influence the volume of the LD. Differences in procedures, such as folding the LD flap for insertion (as in BCS) or spreading the flap over the implant after total mastectomy, can also affect changes in volume. Although it was not possible for us to carry out additional research on this topic, the size of the implants was mostly less than 150 cc and there were no cases of NAC removal or skin excision. Therefore, we concluded that variation in the surgical method was relatively unimportant in the present comparison of LD volume reduction.
The long-term clinical outcomes and satisfaction levels were generally more favorable in patients who did not have implants. When implants were used, there were complications such as capsular contracture and deformity; most patients whose implants were inserted in the dual plane experienced breast animation deformity, and those who complained of severe breast animation deformity, including asymmetry, underwent revision surgery. One-third of patients who received implants reported involuntary movement of the LD, shoulder pain, and/or donor site discomfort even 5 years after surgery, and these results will be presented in more detail in future studies by our research group.
Some challenging parts of this study, which is the last of our serial studies on LD volume change after breast reconstruction using eLD flaps, should be noted. Long-term monitoring for a minimum of 3 years was necessary to document the clinical results, including at least 2 years of observation after the completion of radiotherapy to evaluate its effects. Since it is rare for CT scans to be taken at precisely the same follow-up times due to the varying personal preferences of breast surgeons, it was necessary to include patients who underwent surgery using the same methods and had CT results from the same time points to reduce bias. It was also challenging to recruit a sufficient number of patients, since only 48 of 147 patients met the criteria of an absence of complications such as breast cancer recurrence, infections, seroma, or hematoma. Including more subjects was also difficult due to the recent health insurance policy change covering total mastectomy for breast cancer, as well as changes in the media about breast reconstructive methods. In addition, body weight changes due to various factors, such as the side effects of tamoxifen or adjuvant therapy, were not considered; therefore, another limitation of this study is that weight change was not considered sufficiently in patient selection and the statistical analysis. These factors all led to stronger preferences for breast reconstruction using an implant after total mastectomy instead of reconstruction using an eLD flap.
The overall results of our serial studies, including the current research, show that the average volume reduction of LD was 54.5% when receiving reconstruction with an eLD flap after total mastectomy without POCTx or PORTx, 62.0% when only POCTx was performed, and 69.0% when only PORTx was carried out. Surgeons should also anticipate an average LD volume reduction of 64.5% when POCTx and PORTx are planned postoperatively after BCS with an eLD flap.
Notes
Conflict of interest
Su Bong Nam is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Ethical approval
The study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2021-031) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: KH Song, HY Kim, YJ Jung, SB Nam. Data curation: KH Song, WS Oh, MW Kim, KS Choo, KJ Nam, SB Nam. Formal analysis: JW Lee, MW Kim, YJ Jung, KJ Nam, MS Yun, SB Nam. Methodology: KH Song, JW Lee, DK Jeong, HY Kim, YJ Jung, JH Joo. Project administration: JW Lee, DK Jeong, HY Kim, JH Joo, SB Nam. Visualization: WS Oh, SH Bae, KS Choo, KJ Nam, JH Joo. Writing - original draft: WS Oh, KS Choo, SB Nam. Writing - review & editing: KH Song, WS Oh, JW Lee, MW Kim, SH Bae, MS Yun. All authors read and approved the final manuscript.