Impact of the COVID-19 pandemic on breast surgery and breast reconstruction in a Japanese university hospital setting
Article information
Introduction
Coronavirus disease 2019 (COVID-19) has spread worldwide, and the World Health Organization categorized it as a pandemic on March 11, 2020. The COVID-19 pandemic has had a major impact on hospitals throughout the globe and caused a collapse of medical care in many regions. Although Tokyo did not experience a medical collapse, several major hospitals were affected. There were no restrictions on hospital admission, and all hospital wards were free to admit patients. However, due to surgical restrictions, many surgeries were canceled and surgical admissions were postponed. Patient-initiated suppression of medical visits further decreased the number of patients who were admitted to the hospital.
On April 10, 2020, the Tokyo Metropolitan Government declared a state of emergency. In response, the hospital decided to strictly limit the number of surgical cases in accordance with the COVID-19 surgical triage guidelines issued by the American College of Surgeons. The operating room (OR) restrictions began on April 20, 2020. Only urgent operations were performed, except for those requiring only local anesthesia.
Before the pandemic, the department of breast surgery scheduled up to six surgeries per week, and approximately 250 to 300 breast cancer surgeries were performed annually. Breast surgeons decided to postpone all surgeries beginning in mid-April 2020. In June 2020, the restrictions were relaxed after the lifting of the national emergency declaration, and three operations per week were permitted. In August 2020, the OR schedule reverted to normal.
This study sought to determine how patients requiring surgery for breast cancer or breast reconstruction at the Tokyo Medical University Hospital were affected by the COVID-19 pandemic.
Methods
This retrospective single-center chart review was approved by the Institutional Review Board of Tokyo Medical University. We investigated the treatment of breast cancer patients during the following periods: (1) April to July 2020 (when surgery under general anesthesia was restricted); (2) August to November 2019 (the period after the Allergan recall, discussed later); or (3) April to July 2019 (1 year prior to the survey period in [1]).
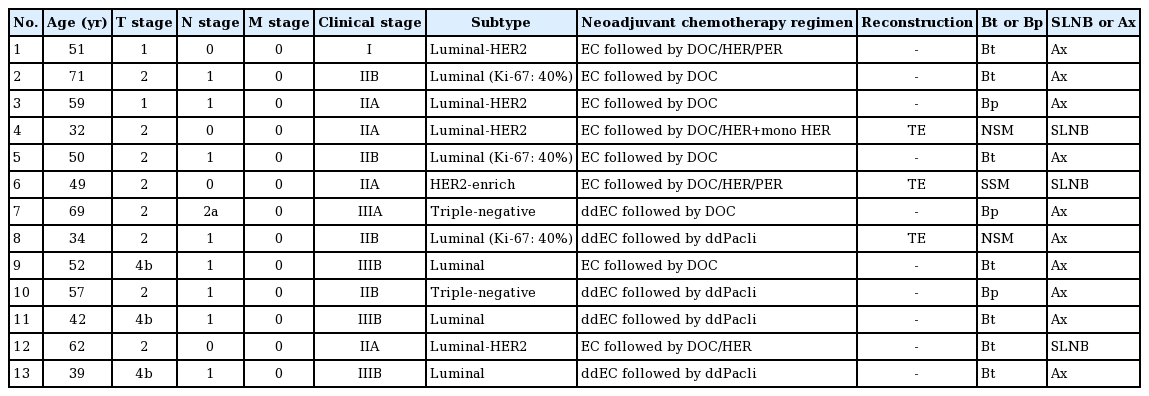
During these periods, patients diagnosed with breast cancer or referred after diagnosis to an outpatient breast clinic, patients referred from outpatient breast clinics to outpatient plastic surgery clinics for breast reconstruction, and patients awaiting breast reconstruction (e.g., autologous tissue reconstruction, implant reconstruction, and nipple-areola reconstruction) at an outpatient plastic surgery clinic were included. The number of patients with breast cancer or undergoing breast reconstruction, the number of surgeries and surgical procedures, the number of postponed surgeries and reasons, and changes in the number of reconstructive procedures were collected from medical records.
Triage and prioritization of breast surgery during the COVID-19 pandemic
The Japan Surgical Society announced a surgical triage protocol under COVID-19 based on the American College of Surgeons protocol on April 14, 2020. We created our own triage for breast cancer based on the Japan Surgical Society protocol. Patients with breast cancer were divided into three categories:
(1) High-priority (requiring urgent treatment)
• Salvage operation for surgical complications, such as hematoma removal or revision of an ischemic autologous flap
• Rapidly growing phyllodes tumor
(2) Medium-priority (treatment delay could worsen outcomes)
• Stage I and II hormone receptor-positive: surgery is postponed and endocrine therapy is administered.
• T2 or N1 hormone receptor-positive human epidermal growth factor receptor-2 (HER2)-negative breast cancer: neoadjuvant chemotherapy is administered as an alternative to endocrine therapy.
• Triple-negative or HER2-positive breast cancer: surgery is considered depending on local conditions.
(3) Low-priority (immediate treatment not required and can be deferred)
• Prophylactic surgery: surgery is postponed.
• Ductal carcinoma in situ: surgery is postponed.
• Re-excision of positive margins and cases of effective neoadjuvant endocrine therapy: surgery is postponed
Triage and prioritization of reconstructive treatment during the COVID-19 pandemic
Triage and prioritization were performed in accordance with the recommendations of the American College of Surgeons and American Society of Plastic Surgeons [1,2]. Only tissue expander insertions were allowed for immediate reconstruction. Patients who requested autologous tissue reconstruction were recommended to undergo immediate two-stage breast reconstruction. All implant replacements, autologous replacements (as the second operation in two-stage reconstruction), and delayed reconstructions were canceled, although this policy was eliminated after the relaxation of the OR restrictions. However, implant replacement was considered for patients whose surgeries had previously been canceled due to the Allergan recall. Nipple-areolar complex (NAC) reconstruction under local anesthesia continued during the pandemic.
Results
A summary of the results is shown in Figs. 1, 2 and Tables 1-4.
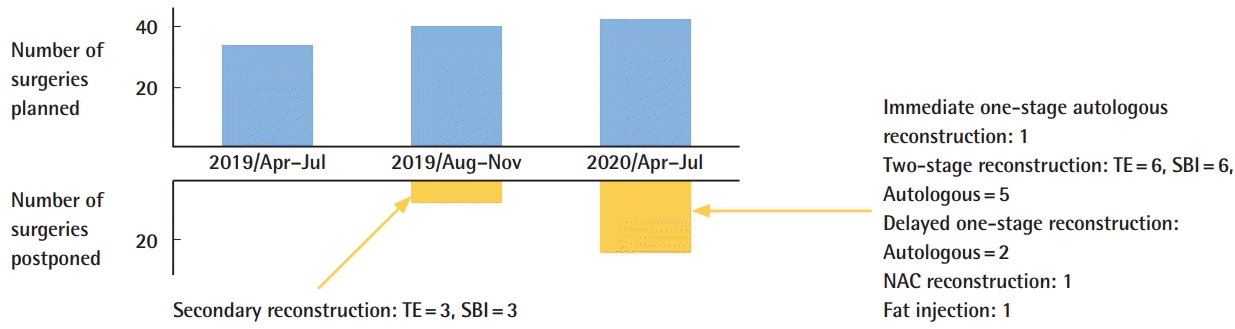
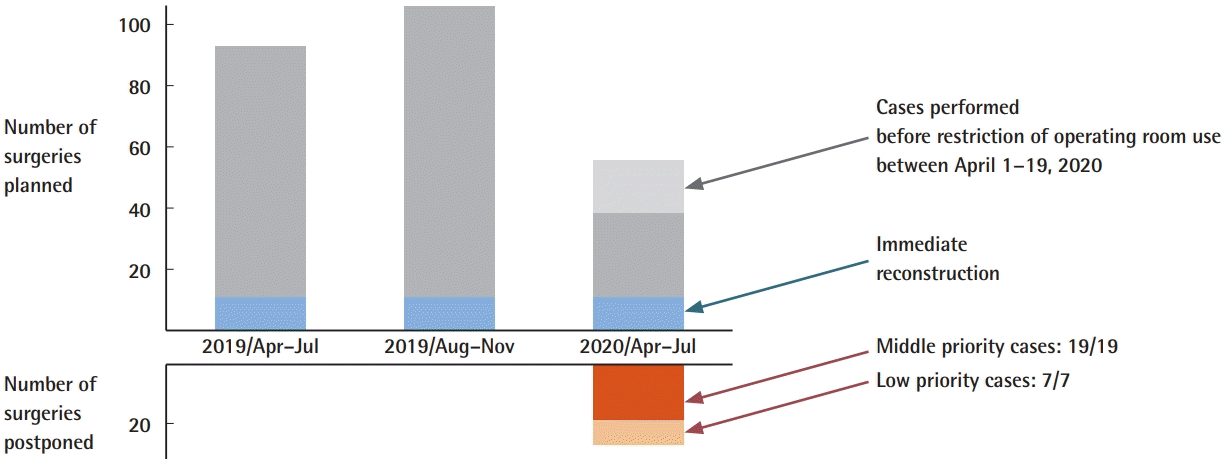
Breast reconstruction postponement in the three periods. TE, tissue expander; SBI, silicone breast implant; NAC, nipple-areolar complex.
Breast cancer and breast reconstruction patients during the pandemic period (April to July 2020)
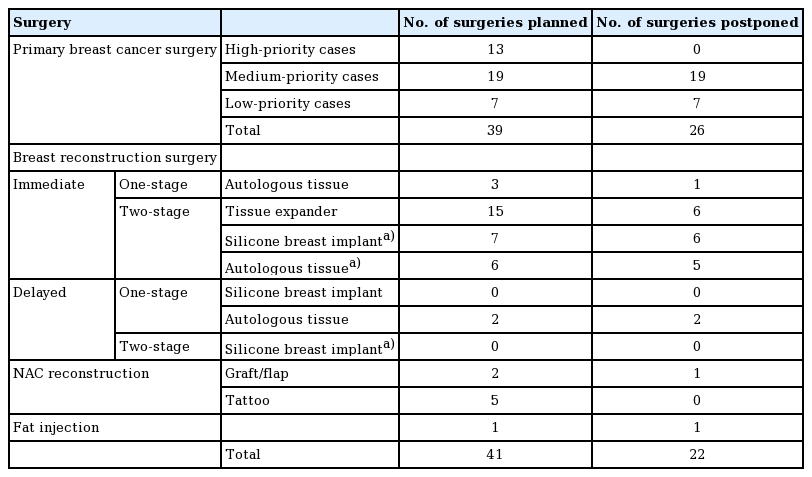
Fifty-six patients were candidates for breast cancer surgery during this period. Seventeen breast cancer surgeries that were planned between April 1 and April 19 were unaffected by the OR restrictions. From April 20 to June 31, 39 breast cancer surgeries were scheduled, but 26 (66.7%) were postponed due to the level of priority (medium or low). Forty-one breast reconstruction surgeries were planned during this period. Seventeen additional patients underwent breast reconstruction but did not have surgery scheduled during the specified period.
Postponed surgeries during the pandemic period
Primary breast cancer surgery was postponed in 26 patients (19 medium-priority patients and seven low-priority patients). All postponed medium-priority patients received neoadjuvant endocrine treatment (oral tamoxifen or aromatase inhibitors) while awaiting rescheduling. The duration of neoadjuvant endocrine therapy was relatively short (less than 1–3 months). Surgeries were performed later in the pandemic period using surgical slots in the plastic surgery department. All surgeries in the low-priority group were postponed once and performed at a later date.
We postponed 22 of 41 breast reconstruction surgeries. Details of the reconstructive procedures are shown in Table 1. Thirteen surgeries were postponed at the surgeon’s request and nine at the patient's request, mostly due to fears of contracting COVID-19 in the hospital.
Surgeries performed during the pandemic period
Surgery was performed in 56 patients during the pandemic period (including 17 patients who underwent surgery before the OR restriction), as follows: total mastectomy in nine patients, partial mastectomy in 29 patients, breast reconstruction in 11 patients, and others (hematoma evacuation and axillary lymph node dissection) in seven patients. Two immediate one-stage breast reconstructions, 11 immediate two-stage breast reconstructions, and six NAC reconstructions were performed.
Oncological outcomes of the triaged breast cancer patients
The triage of patients with breast cancer did not result in any adverse oncological outcomes. No disease progression was observed during the study period in any of the medium-priority patients for whom surgery was postponed (Table 4).
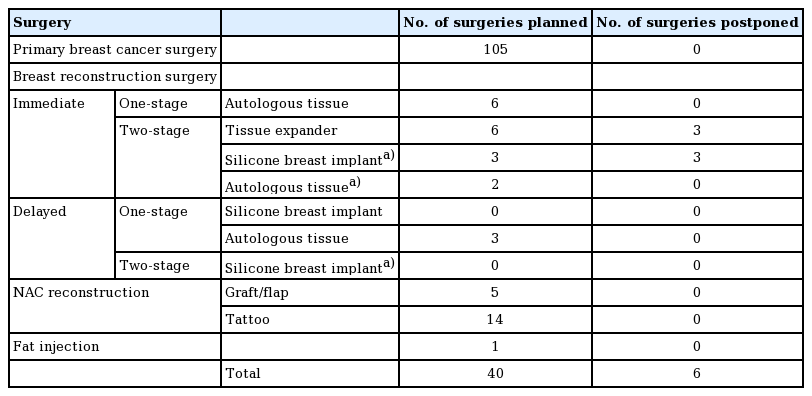
The period after Allergan announced a recall (August to November 2019)
The results for the second period are shown in Table 2. A statement with regard to the significant number of breast implant-associated anaplastic large cell lymphoma cases was issued by Allergan in July 2019. In Japan, the Allergan product was the only approved implant at that time. Six surgeries were postponed due to the Allergan recall. One patient was switched to autologous tissue reconstruction, four awaited implant replacement surgery, and one reconstruction was canceled at the patient's request. COVID-19 clearly had a greater impact on more patients than the Allergan recall in terms of postponed surgeries. However, COVID-19 had only a short-term impact, because the restrictions were lifted after the number of infected individuals decreased.
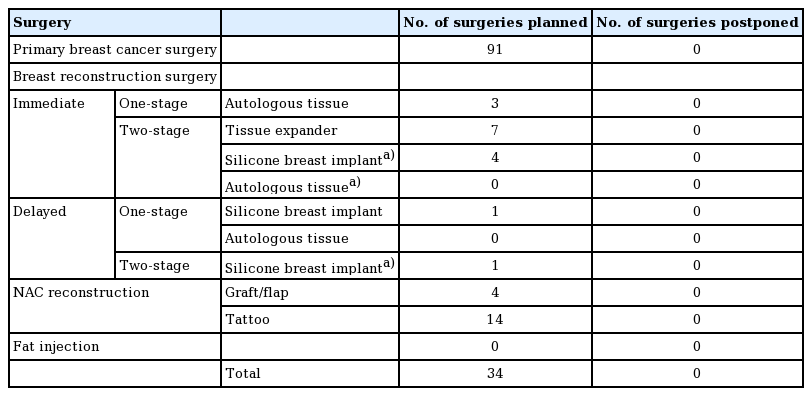
One-year prior to the COVID-19 pandemic period (April to July 2019)
The results for the third period studied in this research are shown in Table 3. No surgeries were postponed during this period.
Discussion
Few studies have described triage methods and reported patient turnover during the COVID-19 pandemic, and none have closely compared the number of surgeries and surgical procedures with those performed before the pandemic [3-5]. The objectives of the triage protocol defined herein were to prevent infection of medical personnel, appropriately allocate medical resources, and avoid affecting patients’ prognoses. The oncological prognoses of the patients included in this study were unaffected; therefore, the response plan was extremely successful. However, differences in race, wealth, and insurance availability vary across countries. Therefore, care must be taken when adapting this triage plan outside of Japan, as triage needs to be flexible and varied to avoid treatment disparities [6-8]. Axillary lymph node dissection was performed in 10 of 13 (76.9%) cases undertriage. These 10 cases were diagnosed with a higher clinical stage and positive axillary lymph nodes at the time of initial diagnosis. Overall, there was a tendency to prioritize oncologically high-risk cases.
Patients in the medium-priority group were the most difficult to manage. All patients in this group received neoadjuvant endocrine therapy during surgery deferral [9,10]. Neoadjuvant endocrine therapy increases the response rate and improves surgical outcomes for hormone receptor-bearing breast cancer [11-13]. Despite its underutilization, this was a management option even before the COVID-19 pandemic. We administered this treatment to 19 patients during surgical postponement, and this triage was successful. No patients had advanced breast cancer due to delayed breast cancer surgery during the COVID-19 crisis, which is similar to the findings from a single hospital in South Korea [14].
Furthermore, the medium-priority group included a patient who requested immediate breast reconstruction. Breast reconstruction plays an important role in the cosmetic aspect of breast cancer surgery; however, it was difficult to prioritize this procedure. During the pandemic, the avoidance of immediate breast reconstruction was preferable because immediate reconstruction is associated with more complications and subsequent reoperation than simple mastectomies [15,16]. Autologous tissue reconstruction, which is particularly time-consuming, was discouraged. According to an opinion survey, 63.4% of breast cancer specialists in Brazil recommended immediate reconstruction, but only 3.4% recommended autologous tissue reconstruction [17]. Some reports have indicated that breast reconstruction during the COVID-19 pandemic was limited to immediate tissue expander insertion and silicone breast implant replacement only [3,4]. Avoiding autologous tissue reconstruction reduces the operation time and hospital stay, and awake surgery has been reported as a method to reduce the operation time [18].
The need for reconstruction makes breast cancer different from other malignancies, making the treatment of breast cancer during a pandemic more complex. After OR restrictions were relaxed, breast surgeons and plastic surgeons recommended the gradual resumption of tissue expander insertion procedures. In July 2020, we were able to perform two primary first-stage autologous tissue reconstructions. Moreover, the number of NAC reconstructions under local anesthesia decreased as compared to the usual number because these operations were not planned during the pandemic period.
The limitation of this study was the relatively small number of patients in this single-center study, which was conducted at a hospital in Tokyo, where the spread of COVID-19 was relatively low by global standards. In addition, a collapse of the medical system did not occur in Tokyo, and medical resources were not significantly constrained, except for the use of ORs.
It is of the utmost importance to strike a balance between appropriate treatment, adherence to the patient's preferences, and the limitations of social and hospital conditions when making treatment decisions. A variety of simulations may be required to prepare for future pandemics [19]. During the COVID-19 pandemic, a significant number of breast cancer and breast reconstruction surgeries had to be postponed. The triage and prioritization plan that we implemented resulted in none of the patients having disease progression during the restriction period, which demonstrates that this novel strategy was appropriate.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
This study was approved by the Institutional Review Board of the Tokyo Medical University (IRB No. T2020-0246) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this study design is a retrospective chart review.
Author contribution
Conceptualization: H Matsumura. Data curation: D Shibata, T Kawate, T Komiya. Project administration: H Matsumura, I Nakamura, T Ishikawa. Writing-original draft: D Shibata. Writing-review & editing: H Matsumura. Approval of final manuscript: all authors.