Axillary Fistula and Scar Contracture due to Uncontrolled Chronic Infection after Trans-Axillary Augmentation Mammaplasty
Article information
Infection after augmentation mammaplasty should not be underestimated. Because if not appropriately treated, it may lead to serious issues such as scarring, wound dehiscence, reinfection and after all, implant loss [1,2]. Generally, even if in severe infection, the course tends to improve with implant removal, antibiotic treatment and other additional surgical procedures like debridement, drainage [2]. But with above treatments it may lead serious secondary complications [2] and we experienced about axillary fistula and scar contracture with limitation of motion due to uncontrolled chronic infection after implant removal.
A 40-year-old woman was hospitalized for soft tissue defect and limited of motion at left axilla. One year before hospitalization, she underwent trans-axillary augmentation mammaplasty at subpectoral plane. Six months after operation, she got bilateral implants removal through approach site, antibiotic treatment due to infection of left breast, not because of implant rupture. And at that time, there were no signs of axillary infection like folliculitis. After implant removal, she got povidone-iodine wet dressing at axilla. After then, there were improvements of other infection signs. However, exudates at approach site appeared and it did not regress and wound dehiscence occurred. Furthermore wound size was not decreased, and consequently, it remained skin and soft tissue defect.
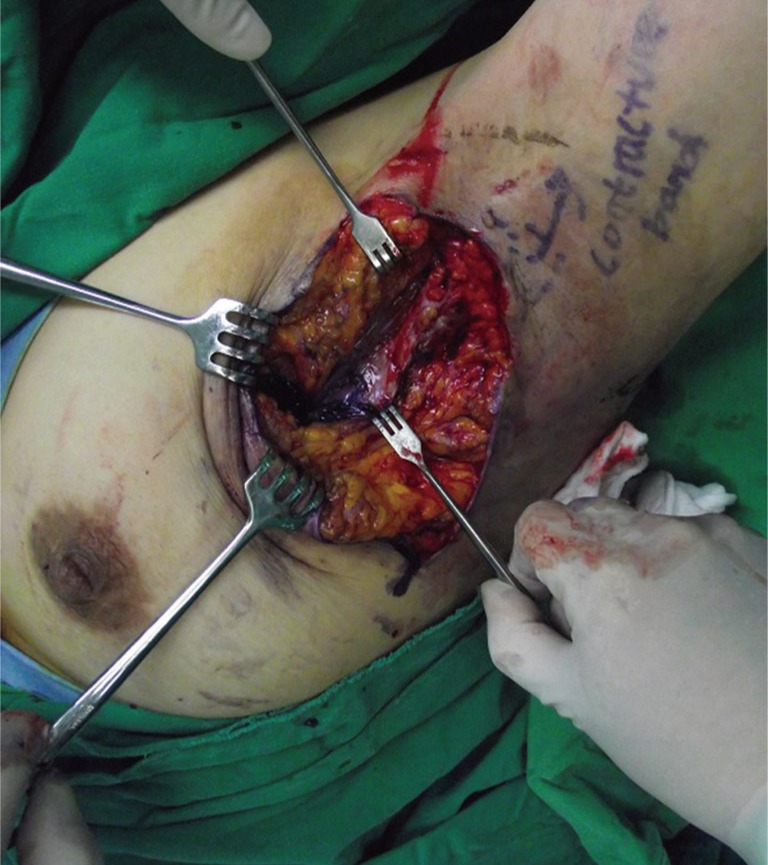
On admission day, we performed physical exams and clinical evaluations. The defect measuring 4×3 cm2 extended fistula of breast in the 7 o'clock region. Scar tissues and contracture band were found around the defect wound (Fig. 1). The active range of motion of abduction was with 70 degrees (Fig. 1) and passive range of motion was with 110 degrees limited due to contracture band.
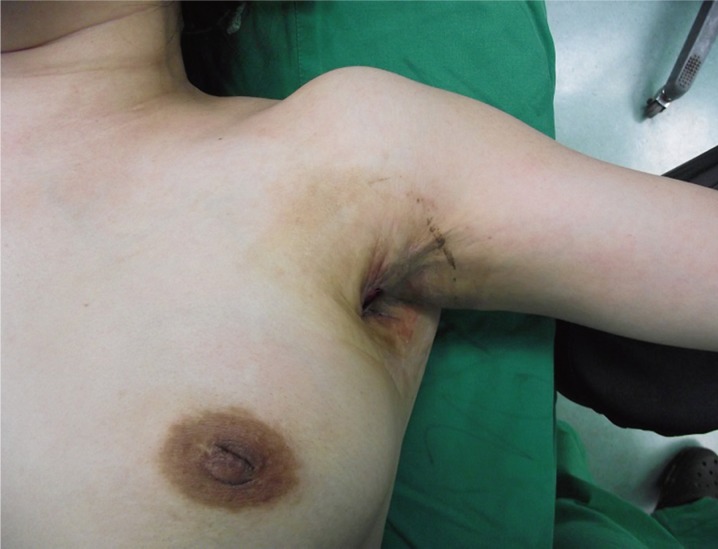
Limitation of motion of left shoulder due to scar contracures is shown. Checked active range of motion is about 70 degrees.
In hematologic exam, it was notable that erythrocyte sedimentation rate (ESR) was significantly elevated while leukocyte and C-reactive protein (CRP) were within normal ranges. We carried out microbiologic exam of pre-operative wound as well as of intra-operative wound, and Methicillin resistant Staphylococcus epidermidis was identified. Also, acid fact bacillus (AFB) stain and Tuberculosis - Polymerase chain reaction (Tb-PCR) examination were performed to rule out mycobacterium infection, and the results were negative. Initially we used second generation cephalosporin as empirical antibiotic and according to the result, we used Vancomycin.
In operation, we checked the state of fistula. The defect was continued to near end of breast parenchyma making a fistula with diagonal direction and the length was 18 cm (Fig. 2).
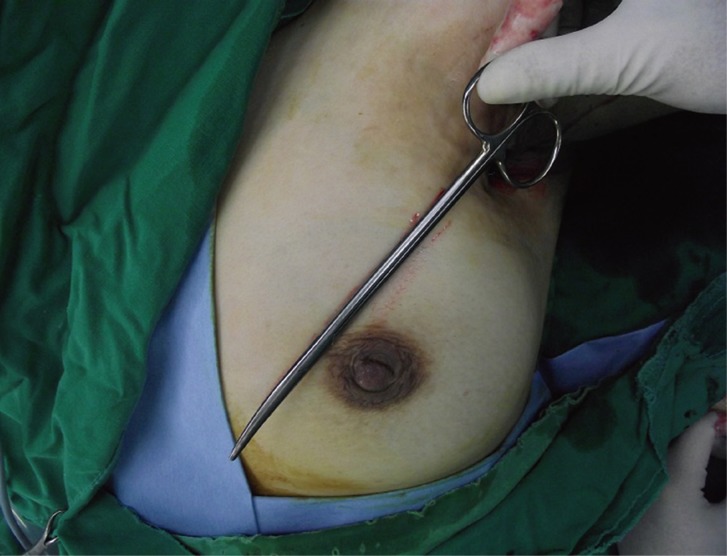
After passive abduction of shoulder, state of defect was checked and it continued the fistula, and the length of fistula was 18 cm.
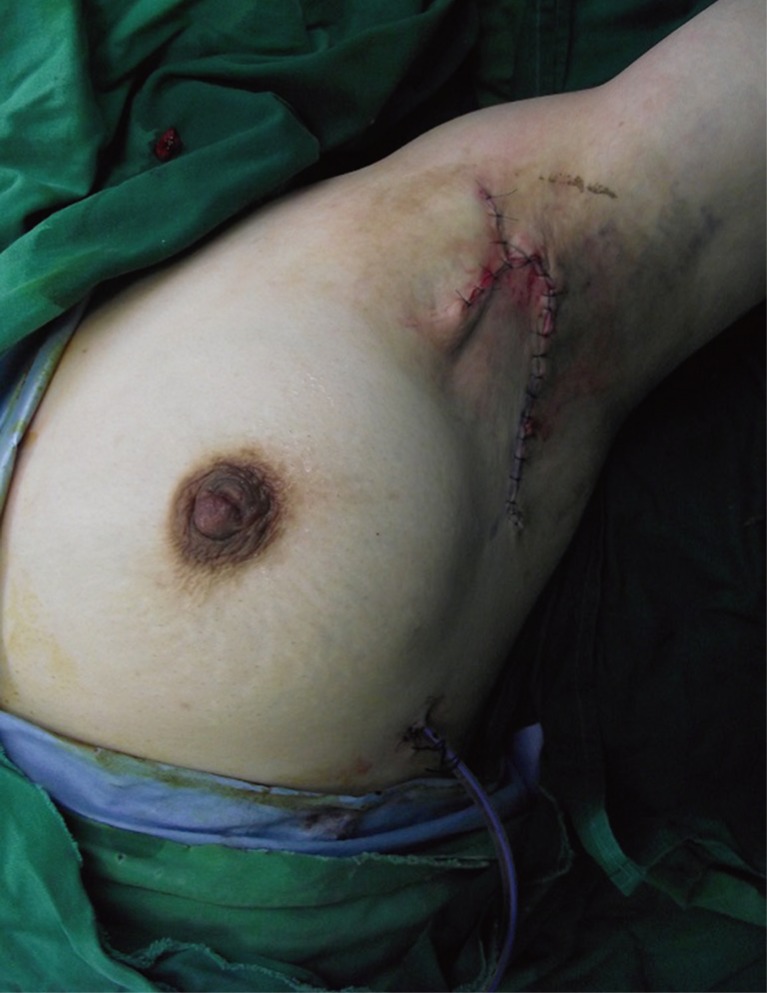
Gentine-violet was applied through the fistula (Fig. 3), and, we performed a debridement by endoscopic guidance. And then, meticulous hemostasis and massive irrigation were carried out. To improve limitation of motion, excision of contracture band and a release procedure were performed. The defect was reconstructed by rotation flap considering the tension of flap with use of lateral aspect of left breast. After then, a drainage tube was placed at the inframammary fold (Fig. 4).
Postoperative wound was clean, and the drain tube was removed 6 days after operation.
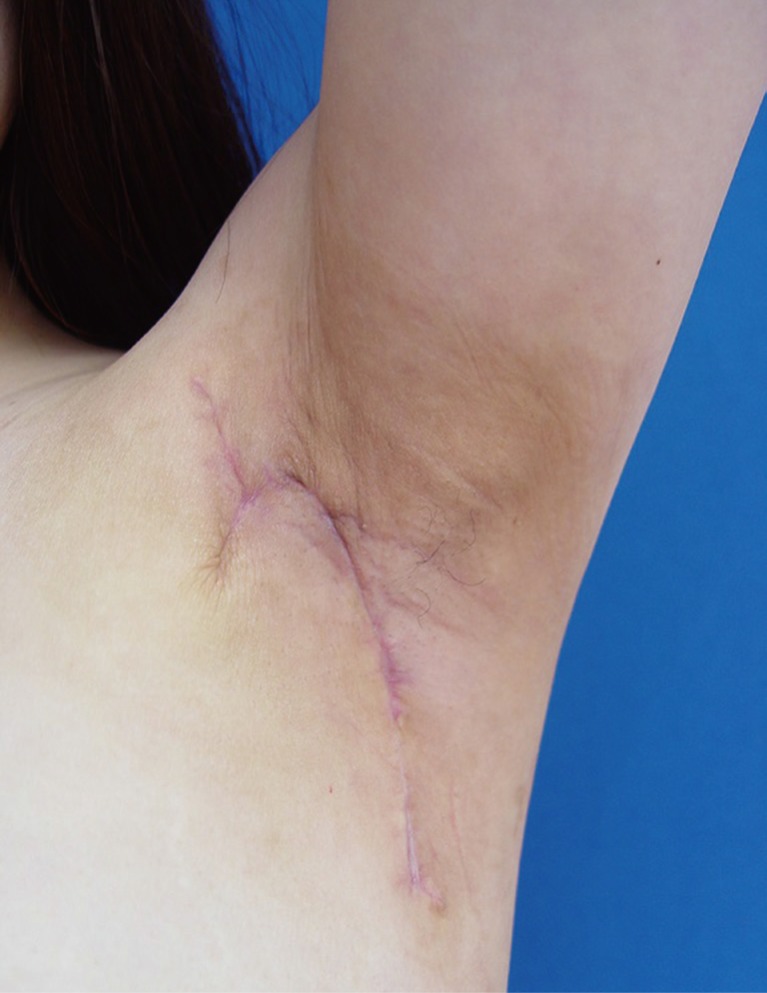
The stitches were removed 9 days after operation and there was no complication. The range of motion of abduction was improved from 70 to 170 degrees. Three weeks after operation, breast ultrasonography was performed to check fluid collection or fistula, and, there was no sign of them. Six months after operation, range of motion was improved to 180 degrees and scar was improved and the patient was satisfied with the result (Fig. 5).
Infection after augmentation mammaplasty has varying degrees of intensity from mild cellulitis or periprosthetic infection to severe infection with pus and systemic symptoms like fever [1], and as presented case, it may lead secondary complications. Important determinants of infection are mainly related with underlying conditions of patient and surgical techniques.
They include patient's history about diabetes or smoking, states of skin or mammary duct, surgeon's aseptic procedures, meticulous hemostasis for prevention of hematoma or seroma, and so on [3]. And these factors influence occurrence of infection by endogenous flora. Most frequently identified pathogen in postoperative infection is Staphylococcus epidermidis, which is normal skin flora [2,3]. And if bacteria colonize around implant, they produce biofilm.
The biofilm causes an inflammation of the host which can lead periprosthetic infection, implant failure, early treatment failure, chronic infection and capsular contracture [2].
As presented case, in uncontrolled chronic periprosthetic infection which is related with biofilm, microbiologic exam of the extracted wound tissues is necessary through operation because bacteria may not be identified by a microbiologic culture of the drained secretion [2].
Also, infections by mycobacterium after augmentation mammaplasty has been increased in recent years and it is related uncontrolled chronic infection, therefore, AFB stain and Tb-PCR tests are necessary, too [3,4].
The typical treatment for infection after augmentation mammaplasty is based on early systemic antibiotic treatment and additional surgical treatment according to the degree of infection [1,2,3]. Especially in severe periprosthetic infection, the cause is infected implant and it may remain serious secondary complications. So, sacrifice such as implant removal is essential in treatment of severe periprosthetic infection [1,3]. And in procedure of implant removal, generally both implants are removed, because adequate symmetry of breast contour is significant for satisfaction of patients [5]. In uncontrolled chronic infection, implant removal is important and surgical debridement is necessary too [2].
In the presented case, since the infection did not improve during 6 months after implant removal and antibiotic treatment, there was a high likelihood that it was chronic infection rather than acute infection. Also significant elevation of ESR implied that it was chronic infection. The possible causes of chronic infection could be antibiotic resistant bacteria, biofilm and remained infected tissue. So, the implants were removed, nevertheless, the uncontrolled infection still existed, and it led to chronic infection. And we resolved the problems about chronic infection, limitation of motion, and defect with elimination of infectious debris around fistula, appropriate antibiotic, release of scar contracture and rotation flap to cover defect area.
As a result of journal reviews about infection after augmentation mammaplasty, there are some reports about secondary complications of infection and they imply that it is hard to be resolved. In conclusion, it is important to control severe or chronic infection after augmentation mammaplasty with active surgical procedures based on appropriate antibiotic treatment, and we report this rare case.
Notes
This article was presented as a poster at the 3rd Research and Reconstructive Forum on May 9-10, 2013, in Daegu, Korea.
No potential conflict of interest relevant to this article was reported.