Applicability and Safety of in Vitro Skin Expansion Using a Skin Bioreactor: A Clinical Trial
Article information
Abstract
Background
Tissue expansion is an effective and valuable technique for the reconstruction of large skin lesions and scars. This study aimed to evaluate the applicability and safety of a newly designed skin expanding bioreactor system for maximizing the graft area and minimizing the donor site area.
Methods
A computer-controlled biaxial skin bioreactor system was used to expand skin in two directions while the culture media was changed daily. The aim was to achieve an expansion speed that enabled the skin to reach twice its original area in two weeks or less. Skin expansion and subsequent grafting were performed for 10 patients, and each patient was followed for 6 months postoperatively for clinical evaluation. Scar evaluation was performed through visual assessment and by using photos.
Results
The average skin expansion rate was 10.54%±6.25%; take rate, 88.89%±11.39%; and contraction rate, 4.2%±2.28% after 6 months. Evaluation of the donor and recipient sites by medical specialists resulted in an average score of 3.5 (out of a potential maximum of 5) at 3 months, and 3.9 at 6 months. The average score for patient satisfaction of the donor site was 6.2 (out of a potential maximum of 10), and an average score of 5.2 was noted for the recipient site. Histological examination performed before and after the skin expansion revealed an increase in porosity of the dermal layer.
Conclusions
This study confirmed the safety and applicability of the in vitro skin bioreactor, and further studies are needed to develop methods for increasing the skin expansion rate.
INTRODUCTION
Skin grafts are required in numerous clinical procedures, such as reconstruction after skin removal because of tumor and ulcers, and correction of contracture or scarring because of burns, accidents, and trauma. Skin graft methods such as traditional sheet skin graft, mesh skin grafts, artificial skin graft, and in vivo skin expansion (for example, tissue expander) are acceptable, but they have several limitations. When a large amount of skin is required for skin graft, secondary damage and scar formation at the donor site cannot be avoided, and also sites selected as donor may not be available because of previous burns and traumas. Although the lack of skin can be overcome by creating holes in the harvested skin to perform a mesh type skin graft, this procedure requires a lengthy recovery time, and secondary problems may develop from cicatricial contractures. The use of artificial skin is possible but is limited because of the need for the patient's own tissue to accompany the artificial tissue, and this procedure is hindered by factors such as cost, non-permanence, vulnerability to infection, and inadequate biocompatibility. In addition, in vivo skin expansion has several disadvantages, including requirement of an additional procedure to perform the skin graft using the patient's tissue obtained, the time-consuming process of procuring sufficient surface area, disruption of the patient's daily life, fibrosis of the tissues, and limited applicable areas.
Therefore, an in vitro skin bioreactor could provide an alternative method that minimizes scarring and damage to the donor site, provides tissue amplification and immunological compatibility, maintains a patient's skin texture, decreases the risk of infection, and is applicable to both partial and full-thickness skin, whilst obtaining a large surface area in a short period of time.
In this study, we used the in vitro skin bioreactor for the correction of cicatricial scar contracture caused by burns and traumas. In addition, we evaluated the clinical applicability and safety of the expanded skin.
METHODS
The in vitro skin bioreactor was designed to expand skin in two directions, after securing it in 4 directions.
The aim was to expand the area about 5% its original size per day over a 2 weeks. The bioreactor had a sensor that was designed to stop the machine when it can't apply force any more. Although it was planned to expand over a two weeks period, in case of any halt at earlier time, it was stopped.
Skin was expanded in Dulbecco's Modified Eagle's Medium containing 10% fetal bovine serum and growth factor supplements with 1% penicillin and streptomycin. The medium was changed daily. The bioreactor chamber was maintained at 37℃, and the CO2 level within the bioreactor was maintained at 5% (Fig. 1).
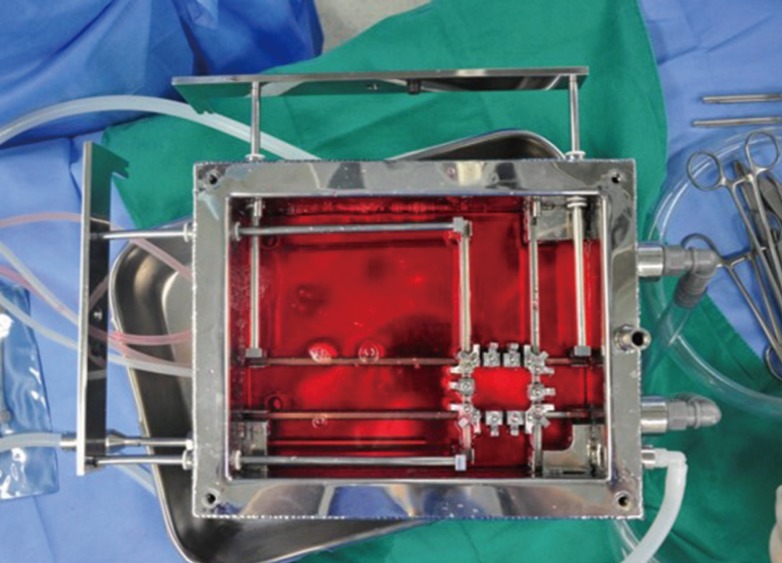
Skin bioreactor with Dulbecco's Modified Eagle's Medium
Skin bioreactor was designed to expand skin in two directions. Skin was expanded in Dulbecco's Modified Eagle's Medium containing 10% fetal bovine serum and growth factor supplements with 1% penicillin and streptomycin. The bioreactor chamber was maintained at 37℃, and the CO2 level within the bioreactor was maintained at 5%.
The study was conducted for 1 year. The age of the patient group varied from 18 to 65 years (average, 48.6 years). Patients were selected from those who needed reconstruction because of cicatricial scar contracture caused by burns and trauma, and who had no psychological or physical limitations that prevented the use of the in vitro skin bioreactor. Patients with mental retardation or cognitive impairment, pregnant or lactating women, patients with serious internal diseases, those taking immunosuppressants or systemic steroids, those who refused to receive a blood transfusion, those allergic to antibiotics, and those who were otherwise deemed unfit to complete the clinical trial in its entirety were excluded from this study. In addition, the size of the surgical site was limited to 100 cm2 and above. Basic information (age, height, weight, and medical history) was recorded, and signed consent was obtained from all participants. Drugs that affect blood coagulation such as, aspirin and anticoagulants, were prohibited 2 weeks prior to admission. An examination in preparation for anesthesia was carried out 2 weeks before the surgery, and clinical photos were obtained to be used for visual assessment after the skin graft. Photos were obtained using equipment that allowed photography under set conditions, and a constant distance and conditions were maintained.
To evaluate the safety and applicability of the procedure, the study was designed using Simon's Two-stage Clinical Trial, without a control group, based on an optimal design. The minimum efficacy limit was set to 60% for the primary evaluation criteria. The expected value of the final take rate was 85%, and significance level was set to 5%. The size of the patient group was calculated using the program PASS2008 (NCSS, Kaysville, UT, USA).
The area of the skin required to correct the cicatricial scar contracture obtained from burns and trauma was calculated before the surgery. The inguinal area was used as the donor site. Full-thickness skin was obtained under local anesthesia and was then transported to, and expanded in, the skin bioreactor in sterile medium.
The area of skin surface was measured by Image J software (1.37v, provided by the National Institutes of Health) and the areas of pre and post expansion were measured in percent through [(Dfinal-Dinitial)=Dinitial].
The expanded skin was used to perform an autograft to correct the cicatricial scar contractures, and conventional dressing methods were used following the skin graft.
Histological examination using hematoxylin and eosin (H&E) staining and Masson's trichrome stain was performed on 5×5-mm samples of each patient's skin after both skin harvest and skin expansion, in order to observe changes before and after the expansion.
After the skin graft, scar evaluation was performed through visual assessment and by using photos taken by medical specialists with a digital camera. The evaluation was performed on the 5-7th days, 2 weeks, 4 weeks, 3 months, and 6 months following the graft. The recipient site was also observed for adverse reactions, and the take rate was evaluated at 1, 2, and 4 weeks after the graft. The contraction rate and scarring were assessed 3 and 6 months after the graft.
Patient satisfaction levels were measured using a survey of the patient's own scar assessment. The donor and recipient sites were assessed separately, and the survey was performed twice at the 3rd and 6th months, using a score from 0 to 10 (Fig. 2).
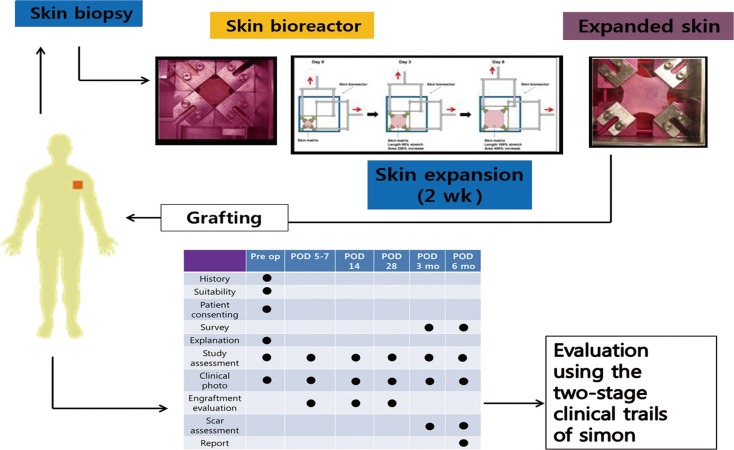
Schematic diagram of the surgery and the evaluation method
After skin harvest, skin expand within 2 weeks. To evaluate the safety and applicability of the procedure, the study was designed using Simon's Two-stage Clinical Trial, without a control group, based on an optimal design. Pre op, preopertaion; POD, postoperation day.
RESULTS
The results showed an average skin expansion rate of 10.54%±6.25% over a period of 11 to 12 days. The analysis on the correlation between dimensional change, take rate and contraction rate according to the duration of extension showed no correlation. The average take rate was 88.89%±11.39% per month after the surgery, and the average contraction rate was 4.2%±2.28% 6 months after the surgery (Table 1, Fig. 3).

Skin expansion rate, expansion period, take rate, and contraction rate after 6 months for each patient before and after the surgery
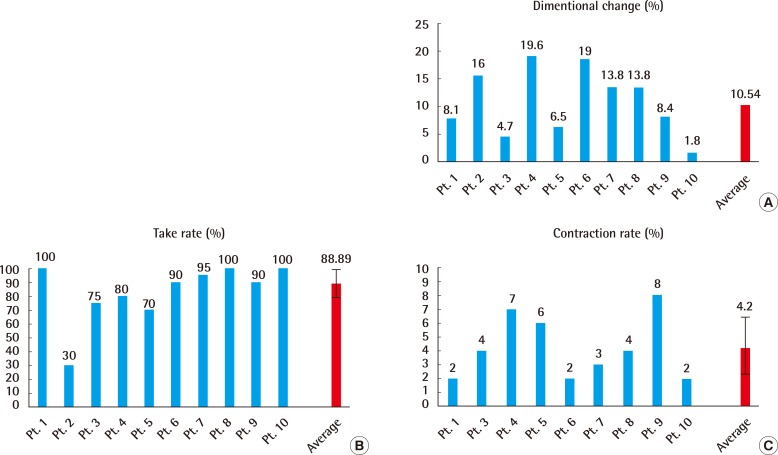
Skin expansion rate, take rate, contraction rate
(A) Skin expansion rate, (B) take rate of grafted skin, (C) contraction rate of the graft site 6 months after surgery. Average skin expansion rate was 10.54%±6.25% over a period of 11-12 days. Average take rate was 88.89%±11.39% per month. Average contraction rate was 4.2%±2.28% 6 months. Pt., patient.
Two plastic surgeons evaluated the photos of the recipient site obtained at 3 and 6 months using the Likert 5-point scar scale. The average score was 3.5 at 3 months, and 3.9 at 6 months (Fig. 4).
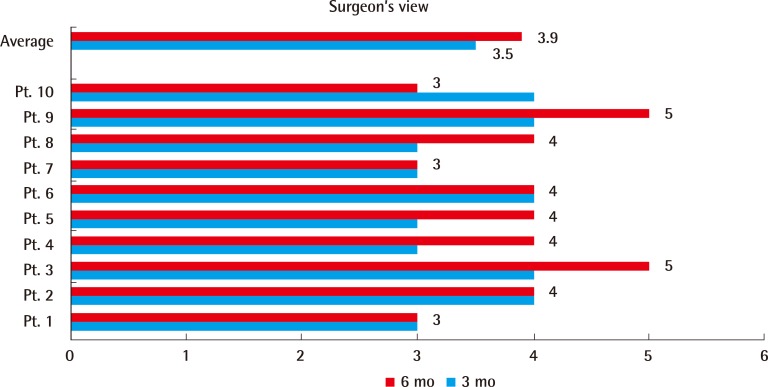
Evaluation of the scar by plastic surgeons
Two plastic surgeons evaluated the photos of the recipient site obtained at 3 and 6 months using the Likert 5-point scar scale. The average score was 3.5 at 3 months, and 3.9 at 6 months. Pt, patient.
Patient satisfaction was evaluated based on the condition of the recipient site, and the feeling of discomfort of the donor at 3 months and 6 months. Satisfaction scores for the donor site were shown to be high, at above 6, while satisfaction scores for the recipient sites were quite low, at 5-6 (Fig. 5).
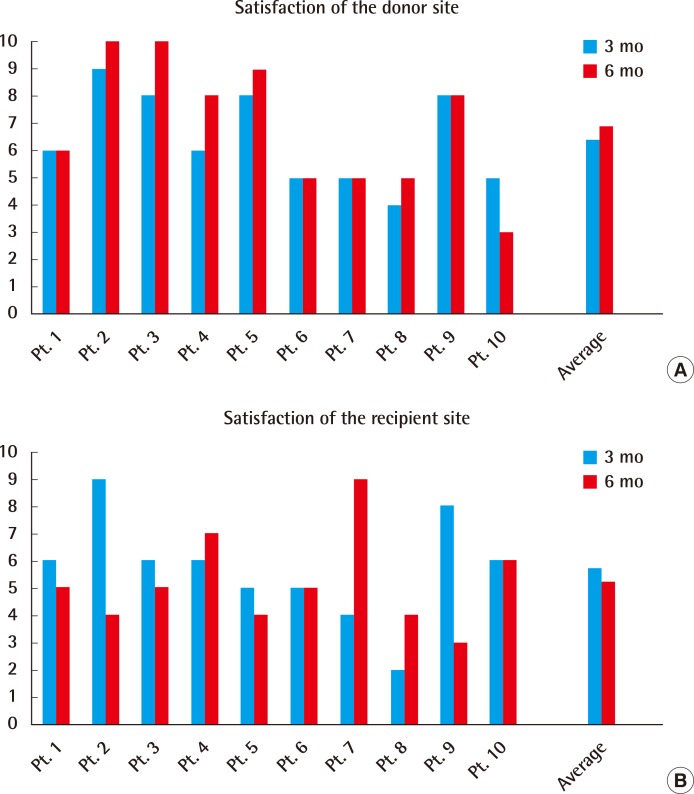
Patient satisfaction at 3 months and 6 months
(A) Satisfaction of the donor site (3 months and 6 months after surgery). (B) Satisfaction of the recipient site (3 months and 6 months after surgery). Patient satisfaction was evaluated based on the condition of the recipient site, and the feeling of discomfort of the donor at 3 months and 6 months. Satisfaction scores for the donor site were shown to be high, at above 6, while satisfaction scores for the recipient sites were quite low, at 5-6.
Histological examination performed before and after skin expansion demonstrated an increase in the porosity of the dermal layer and an uniaxial arrangement of the collagen fibers (Fig. 6).
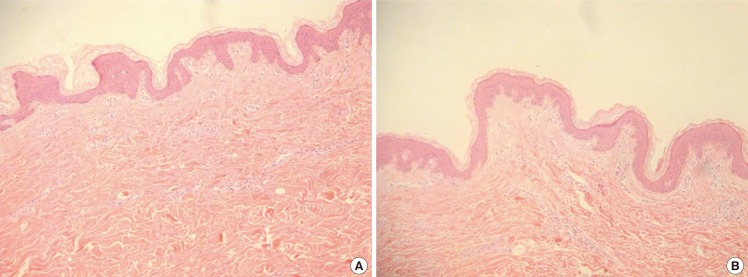
Histomorphological analysis H&E stain
(A) Pre-stretched (×100). (B) Post-stretched (×100). Histological examination performed before and after skin expansion demonstrated an increase in the porosity of the dermal layer and an uniaxial arrangement of the collagen fibers.
Case 1
Case 1 was a 41-year-old female patient with a cicatricial scar contracture from a burn on the neck. The expansion rate was 19.6%, and the skin was expanded for 13 days. The take rate was 80%, and the contraction rate after 6 months was 7%. Patient satisfaction scores for the donor and acceptor sites were both 8 (Fig. 7).
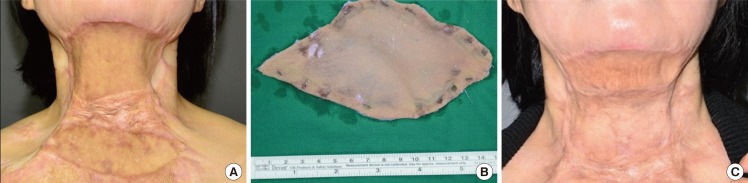
Case 1 photos
(A) Preoperative photo. (B) Expanded skin for 13 days. (C) 9-month follow-up photo. Case 1 was a 41-year-old female patient with a cicatricial scar contracture from a burn on the neck. The expansion rate was 19.6%, and the skin was expanded for 13 days. The take rate was 80%, and the contraction rate after 6 months was 7%. Patient satisfaction scores for the donor and acceptor sites were both 8.
Case 2
Case 2 was a 30-year-old female patient with a cicatricial scar contracture from a burn on the right arm. The expansion rate was 13.8%, and the skin was expanded for 11 days. The take rate was 95%, and the contraction rate after 6 months was 3%. Patient satisfaction scores were 9 for the donor site and 6 for the acceptor site (Fig. 8).
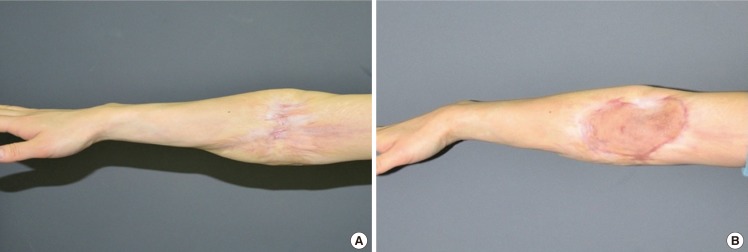
Case 2 photos
(A) Preoperative photo, (B) 8-month follow-up photo. Case 2 was a 30-year-old female patient with a cicatricial scar contracture from a burn on the right arm. The expansion rate was 13.8%, and the skin was expanded for 11 days. The take rate was 95%, and the contraction rate after 6 months was 3%. Patient satisfaction scores were 9 for the donor site and 6 for the acceptor site.
One out of 10 patients was failed in skin graft due to infection and treated through reoperation. This patients' data was excluded from the average value.
DISCUSSION
To mitigate the disadvantages and complications of methods currently in use for soft tissue reconstruction, such as traditional sheet skin graft, mesh skin graft, artificial skin graft, and in vivo skin expansion, we utilized a new method of in vitro skin expansion using a skin bioreactor to perform a skin autograft, and verified its safety and applicability.
Neumann [1] first introduced surgical tissue expansion in 1957. Since then, tissue expansion was believed to be a safe and effective method for treating complex soft tissue defects and scar contractures. Despite the high complication rate of 13% to 40%, tissue expansion has been especially useful for treating scar alopecia and scar contractures. However, tissue expansion has the disadvantage of implant exposure (14.6% cases of exposure were reported during the treatment period by Dotan et al. [2]), and is vulnerable to infection (14.6%). Furthermore, it requires lengthier and more frequent surgical procedures with longer treatment periods, and may cause damage to surrounding structures. To overcome these disadvantages, and also given the advantages of in vitro manipulation, we chose the in vitro skin expansion method and attempted a clinical implementation.
A study on the in vitro skin bioreactor was first reported by Ladd et al. [3] in 2009, while the present study is the first to report its clinical use. In Ladd's study, the foreskin was extracted and expanded uniaxially. The skin length increased by an average of 7.1% following expansion, and the surface area increased by an average of 12.2%. The pore size increased from 94.0 to 159.4 nm on average, representing a 64.5% increase. Histological changes were conserved even after the expansion of the epidermis and the dermis, and the basic structure of the skin was maintained. However, his study was limited because only the foreskin was used, and the study focused solely on skin expansion without completion of the final skin transplant.
Based on these reports, the skin bioreactor was created, and the expanded skin was used to treat patients. The achieved expansion rate of about 12% did not reach the target of doubling in size, but it demonstrated the potential for human skin expansion. Furthermore, the results showed a high take rate of 83%, even after an average of 11.17 days in medium; thus, future applications may be safely achieved. In addition, the low cicatricial contraction rate of 4.3% indicates that skin expansion may be performed to the extent required in future clinical applications.
The donor site was expanded all with the skin from the groin, but it was not expanded in a same period and at a same ratio. This seems to be a result of the difference in each individual skin and it is deemed to be by the differences in the dermis thickness and the composition of the skin, eventhough any actual measurement by histological examination was not conducted in this study. It shall be necessary to carry out an actual histological examination in next studies.
Histological changes during skin expansion were observed in the 2003 study by LoGiudice and Gosain [4]. The thickness of the epidermis stayed the same while the dermis layer quickly thinned. The collagen fibers were quickly redistributed. The subcutaneous fat atrophied, and no change was observed in blood vessel distribution. The muscle layer atrophied during distribution, but returned to normal after the expansion and functioned normally. The distribution of blood vessels dramatically increased during tissue expansion. In the study by Ladd et al. [3] in 2009, the skin maintained its original structure and was not affected by in vitro skin expansion, and the cell viability was conserved. Additionally, an increase in pore size following the expansion was observed, and through this, the diffusion of nutrition could improve and angiogenesis could be induced. Similar results were observed in the present study. H&E and trichrome staining revealed an increase in porosity of the dermal layer. Examination of the microstructure of the expanded skin revealed that the collagen fibers were arranged uniaxially, and Masson's trichrome staining showed a qualitative increase in the porosity of the epidermal layer. However, the increase in porosity was relatively low compared to that reported in previous studies. This is believed to be a result of using a different type of skin. The foreskin was used in the study by Ladd in 2009, whereas inguinal skin was used in the present study, leading to differences in the thickness and structure of the skin (Fig. 6).
Satisfaction of the acceptor site scar was relatively low (5.7 at 3 months and 5.2 at 6 months). This may have been because patients had high expectations of the new treatment method, and subsequently felt let down by clinical results that did not quite meet their expectations. Itchiness, non-smooth surface, color, and patient dissatisfaction (6.4 at 3 months and 6.9 at 6 months) are problems that need to be resolved.
In this study, we confirmed that skin maintains its viability, as well as its expanded surface area and porosity, following expansion in the in vitro bioreactor. Furthermore, the take rate and the contraction rate of the expanded skin were established. Although the safety of the in vitro bioreactor was verified, further studies are required to increase the expansion rate.
Notes
This work was supported by a grant of the Korea Healthcare Technology R&D, Ministry for Health Welfare & Family Affairs, Republic of Korea (A091224) and Regenerative Medicine R&D support by Daegu Metropolitan city.
No potential conflict of interest relevant to this article was reported.