The Preventive Effect of Topical Zafirlukast Instillation for Peri-Implant Capsule Formation in Rabbits
Article information
Abstract
Background
Capsular contracture is the most troublesome complication in breast implant surgery. Although capsule formation can be seen as a normal reaction to a foreign body, it can induce pain, hardness, deformity, and other pathologic problems. Surgical intervention is required in severe cases, but even surgery cannot guarantee a successful outcome without recurrence. This experimental study confirms that single topical administration of leukotriene antagonist zafirlukast (Accolate, Astrazeneca) reduces peri-implant capsule formation and prevents capsular contracture.
Methods
Twelve smooth-surfaced cohesive gel implants were implanted in New Zealand White rabbits. These miniature implants were designed to be identical to currently used products for breast augmentation. The rabbits were divided into 2 groups. In the experimental group (n=6), the implant and normal saline with zafirlukast were inserted in the submuscular pocket. In the control group (n=6), the implant and normal saline alone were used. Two months later, the implants with peri-implant capsule were excised. We evaluated capsule thickness and collagen pattern and performed immunohistochemical staining of myofibroblasts, transforming growth factor (TGF)-β1, 2.
Results
The thickness of the capsules in the experimental group was reduced in both dorsal and ventral directions. The collagen pattern showed parallel alignment with low density, and the number of myofibroblasts as well as the amounts of TGF-β1 and TGF-β2 were reduced in the experimental group.
Conclusions
We suggest that single topical administration of leukotriene antagonist zafirlukast can be helpful in reducing capsule formation and preventing capsular contracture via myofibroblast suppression, modulation of fibroblastic cytokines, and anti-inflammatory effect.
INTRODUCTION
More than 50 years have passed since silicone breast implant surgery was introduced. However, capsular contracture is still a major problem. Capsule formation is a normal physiological response that occurs during the placement of the prosthesis in the body, and it cannot be avoided. A pathological contracture of the implanted capsule results in negative psychological, physical, and economic effects for both the patient and the surgeon. Capsular contracture is treated with surgical methods. Nevertheless, there are cases when recurrence occurs following the surgery, which remains a great concern. At present, many studies are conducted to prevent capsular formation and contracture using drugs rather than surgery. Most surgeons agree that the best treatment for capsular contracture is prevention.
Leukotrienes derived from leukocytes are lipid mediators that play an important role in the pathogenesis of inflammation. In November 1996 the Food and Drug Administration approved the use of zafirlukast (Accolate, Astrazeneca), a leukotriene receptor antagonist, for the treatment of asthma. Furthermore, recent studies have demonstrated the effects of leukotriene receptor antagonists on other inflammatory diseases. Zafirlukast, with its specific anti-inflammatory activity, anti-contractile effect on the smooth muscle, and acceptable general toxicity profile, has been introduced as a useful agent for breast capsular contracture.
In this study, we examined the suppressing effect of zafirlukast on capsular formation and contracture in a rabbit model.
METHODS
Experimental animals
This study was approved by the Animal Review Committee, and it was conducted at the Animal Research Laboratory. Throughout the experimental procedure, the use and management of experimental animals were performed in accordance with Guidelines of the National Institutes of Health for the Care and Use of Laboratory Animals. Experimental animals were twelve 8 weeks old female adult New Zealand White rabbits weighing 2.4-2.6 kg. Rabbits were transferred from the cage to the laboratory. After an approximately 1 month-long adaptation period, they were used for the experiments.
Prosthesis
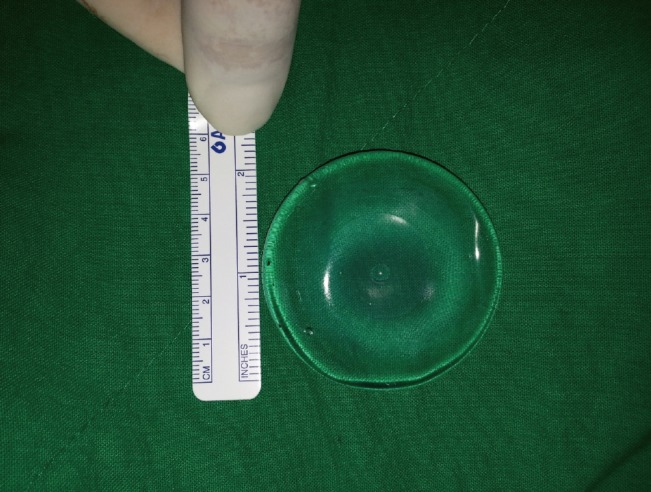
We used a special miniature (5 cm×5 cm×1 cm) cohesive gel silicone implant with a smooth surface designed for use in humans (Hans Biomed Co., Seoul, Korea) (Fig. 1). The prosthesis was sterilized with ethylene oxide according to the manufacturer's instructions and stored in sterile condition until implantation.
Experimental methods
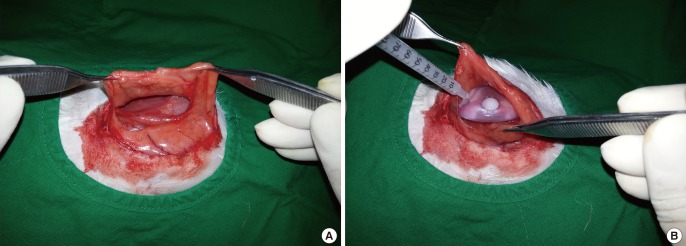
Anesthesia was performed with intramuscular injection of 10 mg/kg of tiletamine/zolazepam (Zotetil, Virbac Korea, Seoul) and 2 mg/kg of xylazine hydrochloride (Rompun, Bayer Korea, Seoul) in the dorsal lumbar muscles. Next, the dorsal region was shaved and disinfected with alcohol and betadine. After injection of lidocaine in the predicted incision line on the skin, an incision with the length of approximately 5 cm was made in the dorsal region. A subpanniculus carnosus dissection exposed the latissimus dorsi muscle. A pocket of sufficient size to accommodate the prosthesis was formed by submuscular dissection. The experimental procedures were performed for 2 groups. In the experimental group (n=6), the implants were inserted with 1 mL of normal saline containing 1.25 mg/kg of zafirlukast. In the control group (n=6), the implants were inserted into the submuscular pockets with 1 mL of normal saline (Fig. 2). We used strict aseptic techniques and fresh powder-free gloves during the insertion of the prosthesis, and the adjacent anatomical structures were well preserved. Upon the completion of the procedures in each group, the muscle layer was sutured using 3-0 vicryl suture, and the skin closure was performed using 3-0 nylon suture. Before recovery from anesthesia, the animals were given intramuscular injections of cefazolin (Cefazolin, Chong Kun Dang Pharmaceutical Co., Seoul, Korea) in the femoral region.
Tissue sampling
Two months after the surgery, the rabbits were sacrificed. Extensive dissection of the adjacent tissue was performed, including the capsule formed around the prosthesis. Twelve implants with peri-implant tissues were harvested en bloc. During the dissection of the adjacent tissue, the dorsal and ventral sides were marked. Thus, tissue samples from each side were obtained separately. Each sample was fixed in buffered (pH 7.4) formalin, embedded in paraffin, and sectioned perpendicularly to full-thickness vertical incisions.
Histology and immunohistochemistry
Tissues fixed in formalin solution were sectioned at a thickness of 4 µm and stained with hematoxylin and eosin (H&E) and Masson's trichrome stain. For immunohistochemical labeling, the sections were placed in a citrate buffer (10% citrate buffer stock in distilled water, pH 6.0) and blocked with 1% horse serum in Tris buffered saline, pH 6.0, for 3 minutes. The sections were incubated with primary antibodies (alpha smooth muscle actin (α-SMA): MS-113-PO, 1A4, Thermo scientific, Fremont, CA, USA, 1:800; transforming growth factor [TGF]-β1: 9016, R&D, Minneapolis, MN, USA, 1:500; TGF-β2: sc-90, Santa Cruz, CA, USA, 1:500) overnight. Antibody binding was detected using polymeric methods (Ultravision LP detection system, TL-125-HD, Thermo scientific). Detection was performed with appropriate secondary antibodies conjugated with horseradish peroxidase, and labeling was visualized with diaminobenzidine.
Statistical analyses
Statistical analysis of thickness of capsules was performed using SigmaPlot (ver. 12.0, Systat Software Inc., Point Richmond, CA, USA). Data are expressed as mean±SD. For convenience of statistical analysis, each immunohistochemical evaluation was scored on a point-based system (0 points for +-, 1 point for 1+, 2 points for 2+, and 3 points for 3+). Statistical analyses were performed using the Mann-Whitney U test for comparing 2 groups. Results were evaluated at P<0.05 significance level.
RESULTS
The thickness of the capsule on the ventral side was 415±71.2 µm in the control group and 248±44.9 µm in the experimental group, whereas the thickness on the dorsal side was 513±93.3 µm in the control group and 346±39.8 µm in the experimental group. In all the animals form the experimental group, the thickness of the capsule was significantly reduced (P=0.002, Mann-Whitney U test) (Figs. 3, 4)
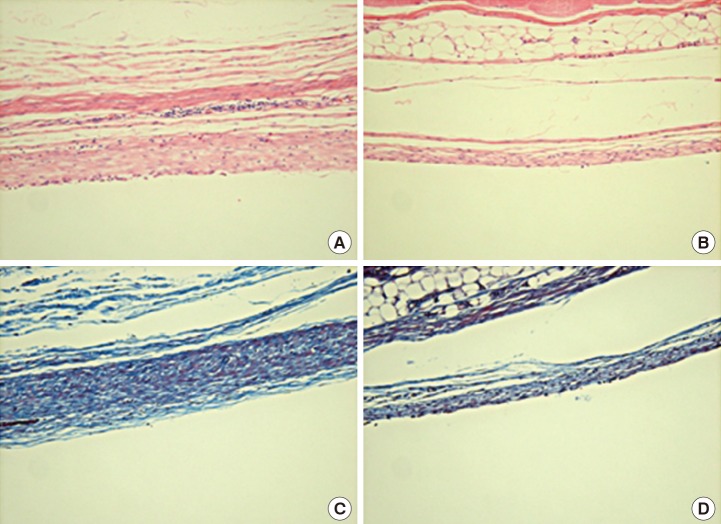
Histology
Compared with (A) the control group (H&E, ×200), (B) the capsule in the experimental group was thinner. Compared with (C) the control group (Masson's trichrome staining, ×200), (D) the collagen fibers in the experimental group were thinner, less dense, and with more parallel alignment.
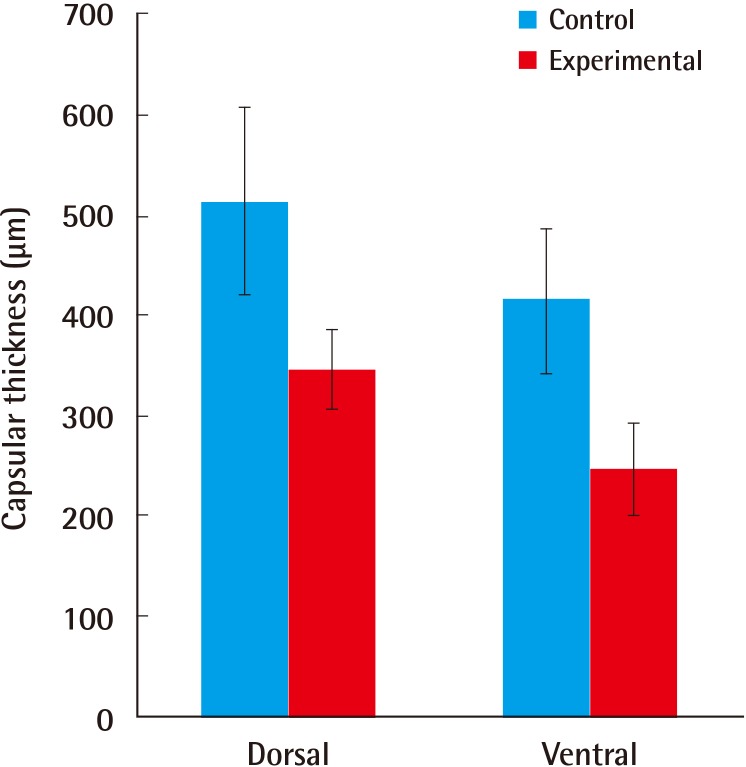
Graphs showing the thickness of the capsule
Dorsal side capsule thickness: 513±93.3 µm and 346±39.8 µm in the control and experimental groups, respectively; ventral side capsule thickness: 415±71.2 µm and 248±44.9 µm, respectively; P=0.002.
Masson's Trichrome staining revealed deposition of collagen around the implants. The collagen fibers in the experimental group were less dense than those in the control group, with a loose and organized pattern (Fig. 3).
For convenience of statistical analysis, intensity of immunohistochemical staining of myofibroblasts, TGF-β1, and TGF-β2 was scored on a point-based system (Table 1). To examine the myofibroblast content in the capsules, the expression of α-SMA was detected by immunohistochemistry. Lower expression of α-SMA was detected in the experimental group (Fig. 5). Furthermore, the levels of TGF-β1 and TGF-β2 in the experimental group were also lower on both the dorsal and ventral sides compared with the control group (P<0.01) (Table 1, Fig. 5).
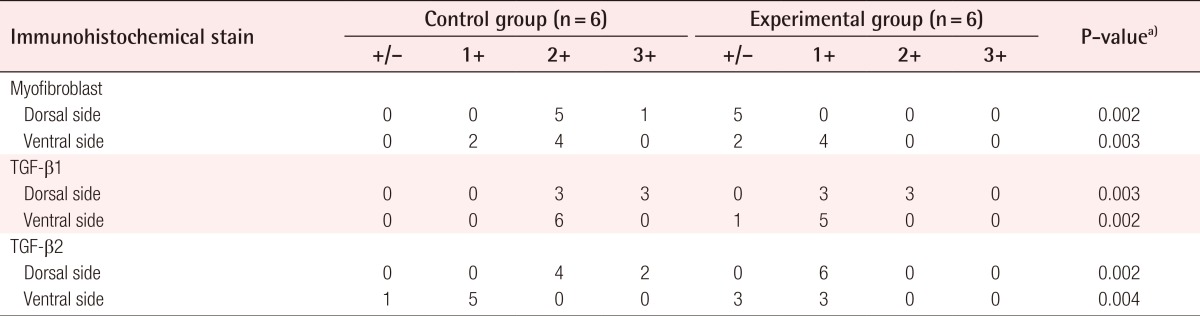
Statistical comparison of the results of immunohistochemical evaluation scored on a point-based system
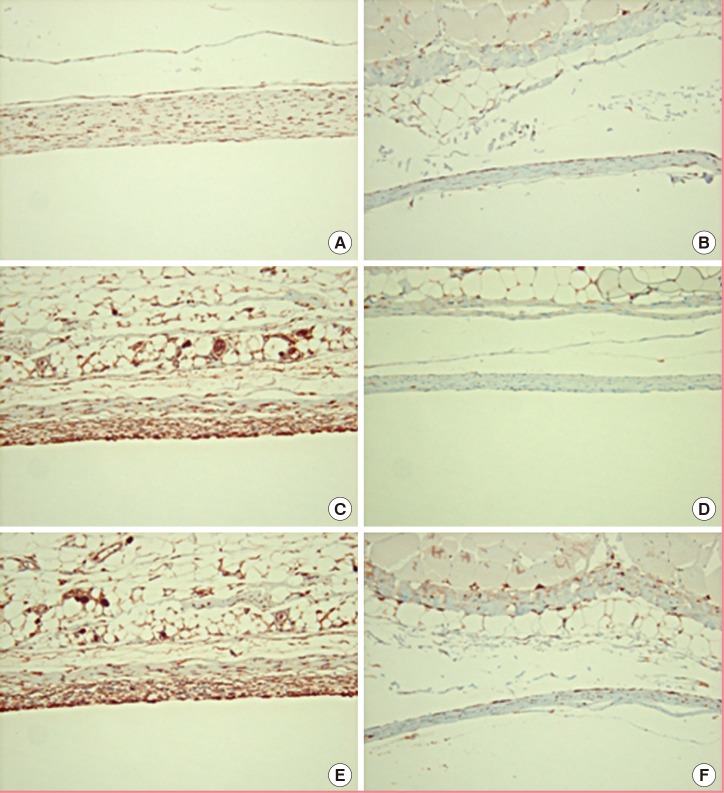
Immunohistochemistry
Compared with (A) the control group (anti-α-SMA immunohistochemical staining, ×200), (B) the myofibroblast ratio of the capsule was lower in the experimental group. Compared with (C) the control group (anti-TGF-β1 immunohistochemical staining, ×200), (D) the TGF-β1 ratio of the capsule was lower in the experimental group. Compared with (E) the control group (anti-TGF-β2 immunohistochemical staining, ×200), (F) the TGF-β2 ratio of the capsule was lower in the experimental group.
DISCUSSION
Capsular contracture is the most serious of the side effects that may occur following mammoplasty. Overall, capsular contracture is characterized by unnatural appearance and changes in position and shape of the prosthesis. Thus, it poses challenging problems for both the patient and the surgeon. Capsule formation may be considered a type of normal reaction to a foreign body. As such, it cannot be avoided following the placement of the prosthesis.
Capsule formation is known to occur via various mechanisms. Representative theories include the subclinical infection theory and hypertrophic scar theory. At present, however, all the phenomena are known to occur in a complicated pattern. Therefore, all possible factors have been considered for the purposes of preventing the formation of capsule. Additional recommended techniques include atraumatic pocket dissection, meticulous hemostasis, soaking of implants in irrigation solution, gloves change before implant handling, and aseptic implant insertion. Manipulations with the prosthesis should be minimized even after its placement. Moreover, the use of systemic antibiotics, cyclosporine A, Vitamin E, steroids, and mesna and minimization of postoperative use of drains have been attempted to minimize the occurrence of capsular contracture. Capsular contracture that occurs despite the application of the above methods is resolved with surgical approaches involving capsule removal and prosthesis replacement. However, the high recurrence rate (up to 54%) limits the use of surgical methods [1]. At present, pharmacological treatment is mainly used to prevent capsular formation and contracture.mechanisms.
Zafirlukast, a leukotriene receptor antagonist, has been used to treat asthma since 1996. Leukotriene is a proinflammatory molecule that is released from mast cells, macrophages, and eosinophils. Leukotriene affects inflammatory responses, supplementation of inflammatory cells, fibrosis, and vascular permeability. Leukotriene receptors are present in several regions of the human body, including the spleen, vascular wall, and smooth muscle. They are associated with systemic diseases such as systemic lupus erythematosus and cystic fibrosis [2]. Zafirlukast is an antagonist of leukotriene receptors D4 and E4. Thus, it inhibits contraction of the smooth muscle layer in the bronchus, airway edema, and increased inflammation and vascular permeability. This eventually leads to reduced infiltration of eosinophils, followed by suppression of pathophysiological processes involved in asthma.
Suppression of inflammatory responses is the rationale behind the usage of leukotriene receptor antagonists for reduction of capsular formation and contracture. Both experimental and clinical studies have reported the effectiveness of zafirlukast in preventing the occurrence of capsular formation [3,4,5,6,7]. In this regard, although little is known about the exact mechanisms, the use of zafirlukast is effective in reducing pain in patients undergoing breast reconstruction surgery, improving deformity of the breast and lowering Baker's grade by 2 points [3,5,8]. According to recent studies, there is a close relationship between the regulation of inflammatory macrophages and fibroblasts and the presence of leukotriene receptors [9,10]. It was postulated previously that only fibroblasts are involved in capsular formation and contracture. Accordingly, only substances regulating myofibroblasts were considered effective in preventing the occurrence of capsular contracture. However, according to the reports of Niessen et al. [11], macrophages play a key role during inflammation and scar formation. Macrophages release many chemokines and cytokines involved in collagen synthesis and proliferation. Not all cellular and immunological responses are solely dependent on fibroblasts. Furthermore, dermal collagen bundle and mast cells are present more abundantly in hypertrophic scars than in scars of general types [12,13,14]. In addition, increased numbers of mast cells are associated with several fibrotic conditions [15,16]. These various results suggest that leukotriene receptor antagonists can reduce the occurrence of capsule formation and capsular contracture.
Evaluation of the capsule with light microscopy was the alignment of the collagen bundle. TGF-β plays a defined role in the synthesis and deposition of collagen and fibrosis that occurs as a part of foreign body response [17]. Shah et al. [18] demonstrated that suppression of expression of TGF-β reduces hypertrophic scars or keloid. Based on the results of the present study, the levels of TGF-β1 and TGF-β2 are reduced in the region around the prosthesis in the experimental group. This reduction reflects the lower incidence of capsular formation. Therefore, targeting the TGF-β pathway might be effective for controlling collagen synthesis and capsular formation.
In the present study, we attempted to demonstrate the efficiency of topical instillation of zafirlukast in preventing the occurrence of capsular contracture. To increase the clinical relevance of this work, we prepared a miniaturized version of the prosthesis that was specifically designed using the current protocol for breast augmentation. Based on the results of several experimental studies that have shown that capsules around breast implants are typically evident within 4 to 6 weeks after implantation, the time period between the surgery and evaluation of the results was set at 8 weeks [19]. Topical use of substances such as antibiotics [20], antineoplastic agents [21,22], and steroids [23,24] to reduce capsular contracture has been reported. Differences in administration routes make it difficult to compare the effectiveness of these drugs. We chose to administer zafirlukast to the submuscular pocket to minimize experimental errors, maximize efficacy, and simplify clinical application. The topical dosage that we used (1.25 mg/kg) had been shown to be optimal in preliminary studies [4,6]. On the histologic examination, a single dose of zafirlukast in the implant pocket significantly reduced capsular formation. Moreover, there were significant decreases in the levels of TGF-β1 and TGF-β2, number of myofibroblasts, and collagen density, as well as the capsular thickness in the experimental group.
Zafirlukast can causes such complications as transient headache, diarrhea, abdominal discomfort, and nausea. However, all the side effects were transient, and none required treatment. It may also cause eosinophilia or vasculitis (Churg-Strauss syndrome). In severe cases, hepatotoxicity may lead to fatal adverse effects such as hepatitis or hepatic failure [3,25]. Therefore, based on the established safety profile in patients with asthma, comprehensive clinical studies are necessary to further explore the potential of zafirlukast and the mechanisms of its action in various clinical conditions.
The results of this study suggest that a single topical administration of zafirlukast is effective in reducing the occurrence of capsular formation. We believe that topical administration of zafirlukast is a simple promising method that can be used to prevent capsular contracture after silicone implant surgery. Thus, further clinical studies are warranted to examine the therapeutic applicability and mode of action of zafirlukast. Moreover, molecular biology studies are also needed to analyze the effects of leukotriene receptor antagonists in suppressing the expression of TGF-β. Other substances that are involved in reducing capsular formation and contracture should also be compared with zafirlukast. Thus, additional studies should be conducted to minimize the occurrence of capsular contracture.
Notes
This article was presented at the 4th Research and Reconstructive forum on April 3-4, 2014 in Busan, Korea.
No potential conflict of interest relevant to this article was reported.