Foreign Body Granulomas after the Use of Dermal Fillers: Pathophysiology, Clinical Appearance, Histologic Features, and Treatment
Article information
Abstract
A foreign body granuloma is a non-allergic chronic inflammatory reaction that is mainly composed of multinucleated giant cells. Foreign body granulomas may occur after the administration of any dermal filler. Factors such as the volume of the injection, impurities present in the fillers, and the physical properties of fillers affect granuloma formation. The formation of granulomas involves five phases: protein adsorption, macrophage adhesion, macrophage fusion, and crosstalk. The clinical and pathologic features of granulomas vary depending on the type of filler that causes them. Foreign body granulomas can be treated effectively with intralesional corticosteroid injections. Surgical excisions of granulomas tend to be incomplete because granulomas have ill-defined borders and moreover, surgical excisions may leave scars and deformities.
INTRODUCTION
The foreign body reaction is the final stage of inflammation and wound healing process which occurs after implantation of various material such as a prosthesis, biomaterials or medical device, which is composed of macrophages and foreign body giant cells [1].
Foreign body granulomas can occur after the injection of dermal fillers, showing various clinical and histologic features depending on the type of injected filler. The reported incidence of foreign body granulomas after filler injection varies. Hyaluronic acid and collagen are the most commonly used filler materials. The incidence of foreign body granulomas after the injection of hyaluronic acid has been reported as 0.02%-0.4%, and the incidence of foreign body granulomas after the injection of bovine collagen has been reported as 0.04%-0.3% [2,3,4].
Both nodules and foreign body granulomas are terms that have been used for palpable lesions noted after filler injections. These terms were once used interchangeably, but they are now used with distinct meanings. In general, foreign body granulomas are not allergic reactions and are often triggered by a systemic bacterial infection, which means that it is not yet possible to predict which patients are at risk [4]. The aim of this article is to present a review of the literature regarding the definition, pathophysiology, clinical features, histology, and treatment of foreign body granulomas according to the type of filler used.
DEFINITION
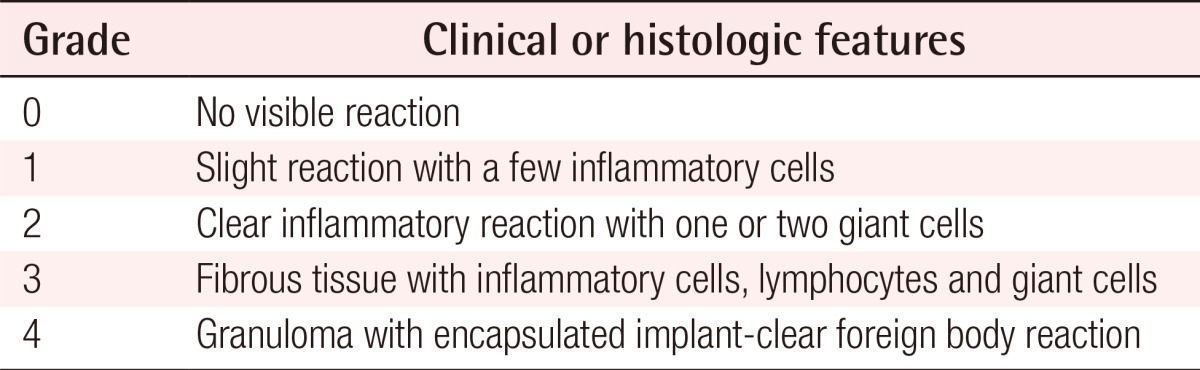
A granuloma is a chronic inflammatory reaction with various etiologies and can be defined as a tumor composed of a collection of immune cells, mainly macrophages [5]. Macrophages are the main cells that constitute granulomas, and in the case of foreign body granulomas, macrophages are activated and fused into multinucleated giant cells [6,7]. These giant cells are characterized by a haphazard arrangement of more than 20 nuclei [5]. The activated macrophages are sometimes called epithelioid cells because their shape is similar to epithelial cells.
Filler-related foreign body granulomas are non-allergic reactions that occur 6-24 months after filler injections and are granulomas mainly composed of multinucleated giant cells [4,5,8].
Duranti et al. [9] classified the severity of foreign body granuloma into four grades in terms of the types and number of cells present (Table 1).
PATHOPHYSIOLOGY
Foreign body granulomas are caused by granulomatous inflammation after the aggregation of macrophages in response to large foreign bodies that cannot be phagocytosed by macrophages [10]. Studies have demonstrated that this process consists of several phases: protein adsorption, macrophage adhesion, macrophage fusion, and crosstalk [1,7,11,12]. In this review, we divided the process into five phases (Fig. 1).
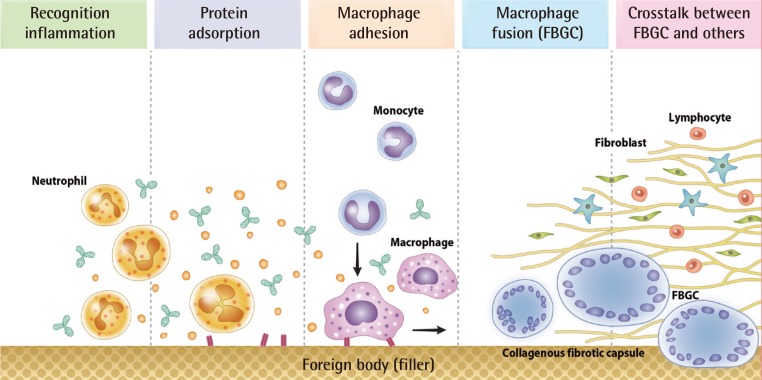
Five phases of the formation of foreign body granulomas
The foreign body response begins rapid neutrophil infiltration, which is with the instantaneous adsorption of various host proteins to the foreign material. The foreign material immediately acquires a layer of host proteins prior to interacting with host cells. Circulating monocytes in the blood slowly migrate to the surrounding tissues and differentiate into macrophages. In cases where the particle volume is greater than the macrophage volume, macrophage aggregation is required and foreign body giant cells are formed. Macrophages secrete factors that recruit and activate fibroblasts, and a fibrous capsule develops around the material. FBGC, foreign body giant cell.
Recognition and inflammation
The foreign body response begins with rapid neutrophil infiltration and the instantaneous adsorption of various host proteins to foreign material. In the host reaction following the implantation of foreign material, circulating polymorphonuclear leukocytes, mainly neutrophils, move into the soft tissue as the first line of defense. Attacking leukocytes release opsonins and soluble factors. If the neutrophils are unable to successfully phagocytose the particle, macrophages take on a more dominant role in the process [10].
Adsorption of plasma proteins
Biomaterials and other foreign material immediately acquire a layer of host proteins prior to interacting with host cells. The presence of adsorbed proteins such as complement, fibronectin, vitronectin, and others modulate the host inflammation and wound healing response [1]. Physical properties such as particle size, surface shape, surface charge, and particle concentration can influence phagocytosis.
Macrophage adhesion
The progression of events in inflammation requires the extravasation and migration of monocytes to the implant site. The guided movement of monocytes occurs in response to chemokines and other chemoattractants. Monocytes circulating in the blood slowly migrate to the surrounding tissues and differentiate into macrophages. Macrophages typically have a life span of several months and replace themselves at a rate of less than 1% per day [12]. When they die, phagocytosed foreign bodies are released, which can cause a recurrence of active, destructive inflammation months or years later [7].
Macrophage fusion (foreign body giant cell formation)
Circulating monocytes are 12-15 µm in diameter; macrophages are 25-30 µm. Macrophages can ingest up to 25% of their volume per hour [12]. Particle size is important in phagocytosis, but size is not the sole determinant of effective phagocytosis. In the case where the particle volume is greater than the volume of a macrophage, macrophage aggregation is required and foreign body giant cells are formed. When multiple macrophages meet each other to fuse, all cells need a stimulus such as interleukin-4 or interleukin-13 [1].
Crosstalk between foreign body giant cell and others
Macrophages and foreign body giant cells secrete an array of inflammatory mediators. They are capable of secreting growth and angiogenic factors that are important in the regulation of fibroproliferation. Macrophages secrete factors that recruit and activate fibroblasts, and a fibrous capsule develops around the foreign material. Lymphocytes play a critical role in the foreign body reaction. Both direct (juxtacrine) and indirect (paracrine) mechanisms of interaction between lymphocytes and macrophages occur at the tissue/material interface [1].
However, this sequence of events does not accurately describe the formulation of foreign body granulomas caused by small-particle fillers or silicone fluid, because small-particle and fluid fillers are easily phagocytosed by macrophages [13,14]. Foreign body granulomas sometimes simultaneously occur in multiple sites if filler was injected at multiple sites; however, additional theories have attempted to explain their occurrence [4]. It is well known that macrophages act as memory cells, which underlies a distinct proposed mechanism for macrophage action [5]. Lemperle et al. [4,15] proposed a hypothesis that macrophages remember small, phagocytosed chemical structures and stimulations such as severe systemic infection can trigger giant cell formation and foreign body granuloma formation [15,16,17].
FACTORS AFFECTING THE OCCURRENCE OF FOREIGN BODY GRANULOMAS
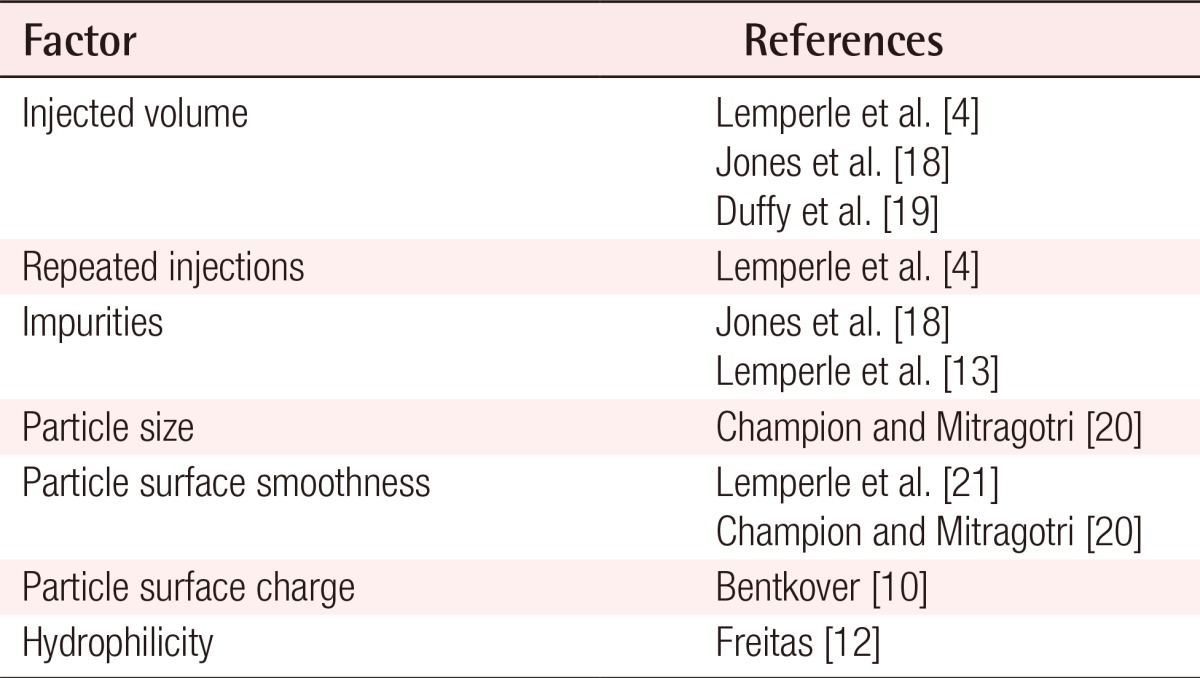
Bentkover [10] described the factors that affect phagocytosis based on the hypothesis that granulomas are formed when there are filler particles that cannot be phagocytosed by macrophages. Lemperle et al. [4] also described the factors that affect the formation of foreign body granulomas. Among these factors, the particle surface and the presence of impurities stood out as particularly relevant: when improved products were used, a lower occurrence rate of foreign body granulomas was noted (Table 2) [4,10,12,13,18,19,20,21].
CLINICAL FEATURES OF FOREIGN BODY GRANULOMAS
Lemperle et al. [4,8] divided foreign body granulomas into three types based on clinical features: cystic granulomas, edematous granulomas, and sclerosing granulomas. The type of foreign body granulomas primarily depends on the type of filler used, but this is not always the case. Moreover, mixed type foreign body granulomas sometimes occur.
FEATURES OF FOREIGN BODY GRANULOMAS ACCORDING TO FILLER MATERIALS
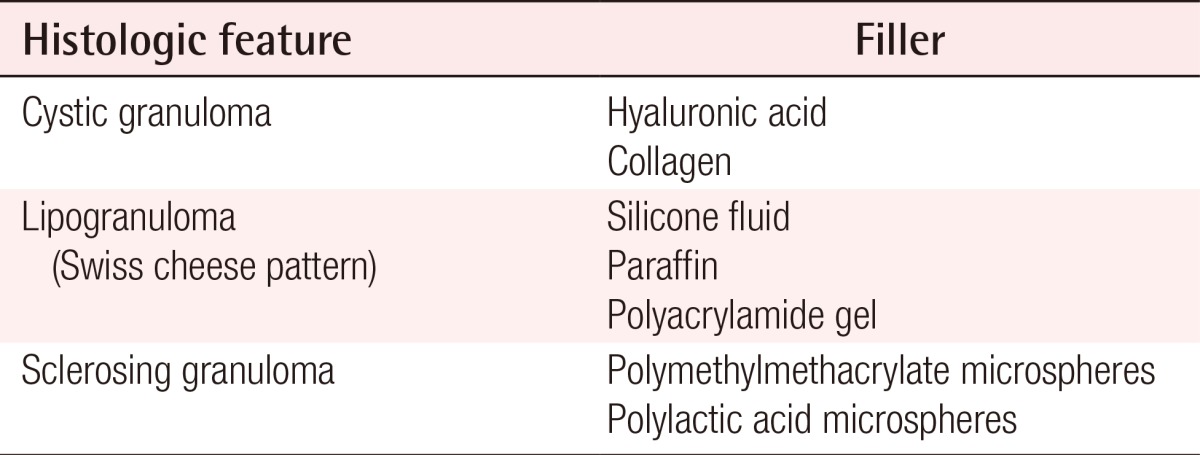
The histologic features of foreign body granulomas vary depending on the filler used (Table 3).
Hyaluronic acid
Hyaluronic acids normally present in the human body have a half-life of 1-2 days. However, hyaluronic acids used as fillers are modified to add artificial cross-linking, which allows them to maintain their volume for more than six months in the human body. Clinically, foreign body granulomas caused by hyaluronic acids mainly appear as cystic granulomas. A process of encapsulation occurs that prevents the absorption of injected material into the surrounding tissues, resulting in the development of a sterile abscess. Histologically, palisaded granulomatous tissue mainly composed of giant cells and macrophages is usually discovered [2].
Collagen
As with hyaluronic acids, foreign body granulomas caused by collagen fillers mainly show the characteristics of cystic granulomas. The reported incidence rate was 0.1%-1.3% in one study [3] and 4.5% in another [22]. They generally show clinical features similar to abscesses and contain many giant cells and macrophages [3,4].
Silicone fluid
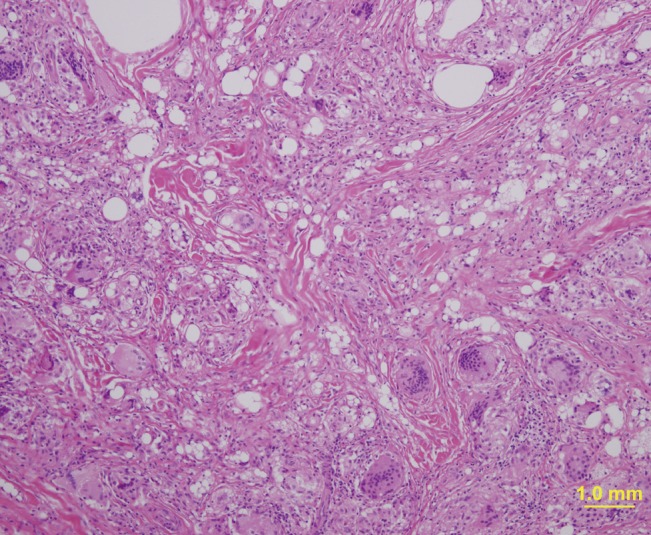
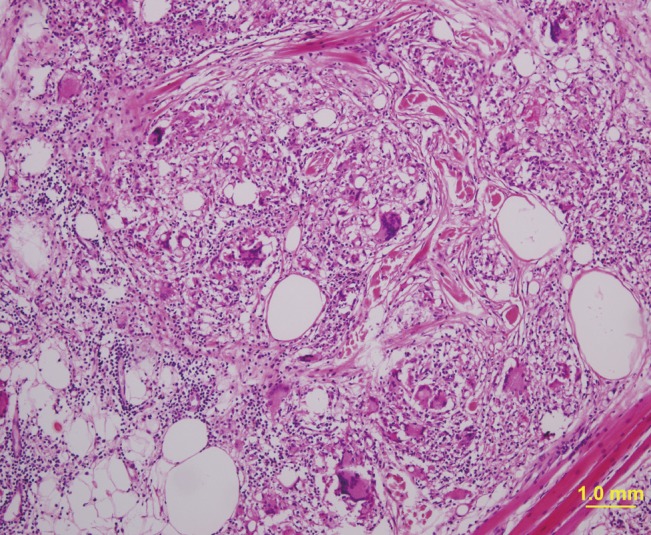
Injectable medical-grade silicone fluid was developed and introduced in the late 1950s and advanced silicone gel fillers are now available for off-label use. However, in the past, non-medical-grade silicone fluid was also used for augmentation in many patients. Both medical-grade and non-medical grade silicone fluid can induce foreign body granulomas. Foreign body granulomas may even occur 10 to 15 years after the injection of silicone fluids with a high rate of incidence. Relatively soft and fixed nodules are palpable and swelling-like symptoms occur, accompanied by redness. Lipogranulomas caused by silicone fluids usually show vacuoles of various sizes with diameters of 1-30 µm, surrounded by lymphocytes, plasma cells, eosinophils, and scattered giant cells (Fig. 2) [14,23]. Similar findings have been observed in silicone gel bleedings among patients who received breast augmentation with cohesive gels.
Paraffin
In the past, paraffin injection was often performed in augmentations of the nose, forehead, and breasts [24]. However, sclerosing granulomas have occurred up to 20 years after paraffin injections, and many patients currently suffer from paraffin-related granulomas [25]. Paraffinomas generally result from the injection of oily substances containing long-chain acyclic hydrocarbons such as mineral oil [3]. It usually manifests as irregular plaque-like indurations of the skin. Macroscopically, excised specimens are white or grayish-white; microscopically, the granulomatous inflammation appears to have multiple, clear vacuoles, resulting in the so-called "Swiss cheese" pattern [26]. The clinical and histological features of paraffin granulomas are similar to those of granulomas due to silicone fluids (Fig. 3).
Polyacrylamide gels
Symptoms like pain, redness, and swelling after injections with Aquamid (Contura International S.A., Copenhagen, Denmark) should be considered as suspected infections and should never be treated with corticosteroids or non-steroidal anti-inflammatory drugs, but instead should be treated with high-dose, broad-spectrum antibiotics. Polyacrylamide gels have been commonly injected in large quantities for breast and buttock augmentation in China and Russia. The clinical appearance and histologic findings associated with polyacrylamide gel granulomas are similar to those of granulomas caused by silicone fluids. Because Aquamid is highly biocompatible and does not cause cellular ingrowth or capsule formation, the migration of the polyacrylamide gel sometimes occurs in patients who have loose connective tissue [4,27]. However, it can be removed up to 10 years after the initial injection by needle aspiration or puncturing and squeezing [28].
Polymethylmethacrylate microspheres
Artecoll (Rofil Medical B.V., Breda, Netherlands) is a mixture of bovine collagen and polymethylmethacrylate (PMMA) microspheres that have a smooth surface and diameter ranging between 30-42 µm. Until recently, Artecoll was commonly used. When foreign body granulomas occur in patients who have been treated with Artecoll, they generally show the features of sclerosing granulomas. Several months after the filler injection, they appear as hard and bluish nodules with congested dermal capillaries on their surfaces. Pathologically, abnormally distributed microspheres are found as empty vacuoles with even sizes and shapes. The spaces between the vacuoles are filled with multinucleated giant cells, macrophages, fibroblasts, and collagen fibers produced by fibroblasts [23].
Polylactic acid microspheres
New-Fill/Sculptra (Dermik Laboratories, Berwyn, PA, USA), a polymer of polylactic acid (PLA), is usually diluted with sterile water several hours before injection. Micro- or macro-nodular cystic reactions and inflammatory nodules have been observed. The incidence of nodules is more frequent with intradermal implantation, with high concentrations of PLA, when it is used in highly mobile areas like in periorbital and dorsal skin [29]. However, PLA microspheres used as fillers sometimes cause foreign body granulomas similar to those caused by suture materials made of PLA. PLA-related granulomas appear 6-24 months after injection and last for 2-5 years if not treated. PLA mainly produces sclerosing granulomas like other particulate fillers; therefore, the histologic findings associated with PLA-related granulomas are similar to those associated with other particulate fillers. They may disappear with corticosteroid injections. Using polarized light microscopy, quite characteristic birefringence of the cystic spaces can be observed [4].
Calcium hydroxyapatite microspheres
Calcium hydroxyapatite is the main component of bone and teeth and is highly biocompatible; therefore; it causes little tissue ingrowth and does not cause granuloma formation. Lumps in the skin, especially in the lip, after calcium hydroxyapatite injection are usually nodules rather than foreign body granulomas; therefore, are not susceptible to steroid injection therapy [30].
DIFFERENTIAL DIAGNOSIS
Nodules
A nodule is a single lump that occurs at the injection site a few weeks after the filler injection. Most nodules are caused by technical errors and usually occur around the mouth and eyes when dermal fillers are injected superficially. Foreign body granulomas are different from nodules in that the size of the granuloma becomes larger than the volume that was injected, and granulomas develop simultaneously at multiple sites of injection [4]. Nodules are evenly sized and whiter in coloration than granulomas. They are harder than foreign body granulomas and show little cellular reaction; thus, intralesional steroid injections are a relatively difficult and ineffective treatment. However, nodules can be treated successfully by excision.
Delayed hypersensitivity
Delayed hypersensitivity reactions usually occur one month after filler injections and differ from foreign body granulomas regarding the time of onset and treatment strategies [3,31]. These reactions usually resolve spontaneously within one year without any sequelae, but oral steroids may be helpful [32]. Dermal fillers containing hyaluronic acid or collagen may induce delayed hypersensitivity reactions [33,34]. To lower the incidence rate of delayed hypersensitivity reactions, a single or double skin test is recommended before the injection of collagen fillers [35,36]. A positive skin test, erythema, edema, an indurated papule, a nodule, and/or itching are characteristic clinical features of delayed hypersensitivity [35].
TREATMENT
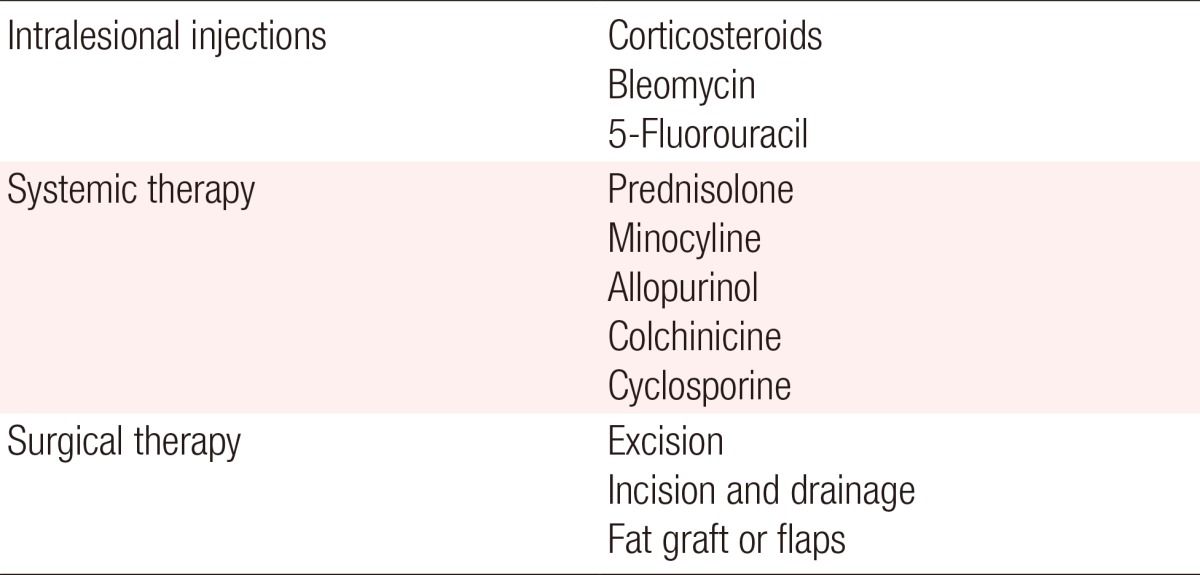
Intralesional injections
The primary treatment of foreign body granulomas caused by fillers is intralesional corticosteroid injections. The local injection of corticosteroids is known to interfere with the activities of fibroblasts, macrophages, giant cells, and with collagen synthesis [37,38]. When performing intralesional steroid injections, it is preferable to inject a high dose of triamcinolone mixed with lidocaine to prevent recurrence. Many reports have successfully treated foreign body granulomas caused by Artecoll [39,40], hyaluronic acids [41,42] and Matridex (Biopolymer GmbH, Montabaur, Germany) [43] using intralesional steroid injections.
Bleomycin has been injected into keloids or hypertrophic scars, and it may work successfully in the treatment of foreign body granulomas [44,45]. In addition, 5-Fluorouracil, an antimitotic agent, has been used in intralesional injections to treat foreign body granulomas [38].
We recommend a 0.5 mL or 1 mL insulin syringe with a 30-gauge needle for intralesional injections. A smaller diameter syringe allows the resistance of the granuloma to be sensed, which helps prevent corticosteroid-induced dermal atrophy. The preferred technique is to inject a small amount of drugs gradually, moving from the periphery to the central area, as granulomas tend to spread into the surrounding tissue in a finger-like pattern.
Systemic therapy
Systemic steroid therapy can be used for recurring foreign body granulomas. When systemic therapy is used, the doses of steroids should be higher than those used in local injections are. The use of oral prednisone at a starting dose and maintenance dose of 30 mg/day and 60 mg/day, respectively, is recommended to prevent the recurrence of granulomas [4].
Minocycline combined with oral or intralesional steroids is effective in treating widespread inflammatory granulomas [46]. It has also been reported that silicone granulomas have successfully been treated with minocycline only [46,47,48]. In addition, the systemic treatment of foreign body granulomas using allopurinol [49,50], colchicines [51], and cyclosporine [52] has been reported.
Surgical therapy
The excision of foreign body granulomas is not a therapy of first choice because the complete removal of a granuloma is impossible in many cases. Genuine granulomas are invasive and have non-confined borders with the surrounding tissue [8]. There are also possibilities of complications such as abscess or fistula formation after surgery in granulomas that become induced by silicone fluid or acrylamide gel [53]. In the case of an obvious sterile abscess, incision and drainage of the abscess is an effective treatment [8]. The excision of a localized sclerosing granuloma can be carefully attempted and subsequent deformities can be corrected using fat grafts or flaps (Table 4).
CONCLUSIONS
In the treatment of foreign body granulomas, it is necessary to consider intralesional steroid injection as a primary treatment option, because most foreign body granulomas can be treated successfully using intralesional steroid injection. Furthermore, a differential diagnosis should be performed to distinguish granulomas from nodules, and appropriate treatment in accordance with the type and cause of granuloma is required.
To prevent granulomas, injecting fillers into the subcutaneous fat layer are considered safer than injecting them into the dermal layer [54]. This is because the skin has very strong immune function and actively performs foreign body reactions [4].
Notes
We are grateful to Prof. Kwan Hyun Youn for his high-quality medical illustrations.
No potential conflict of interest relevant to this article was reported.