Using Local Flaps in a Chest Wall Reconstruction after Mastectomy for Locally Advanced Breast Cancer
Article information
Abstract
Background
Surgical ablation for locally advanced breast cancer results in large chest wall defects, which can then be managed with local flaps or skin grafts. The purpose of this article is to evaluate the outcomes of three types of local skin flaps.
Methods
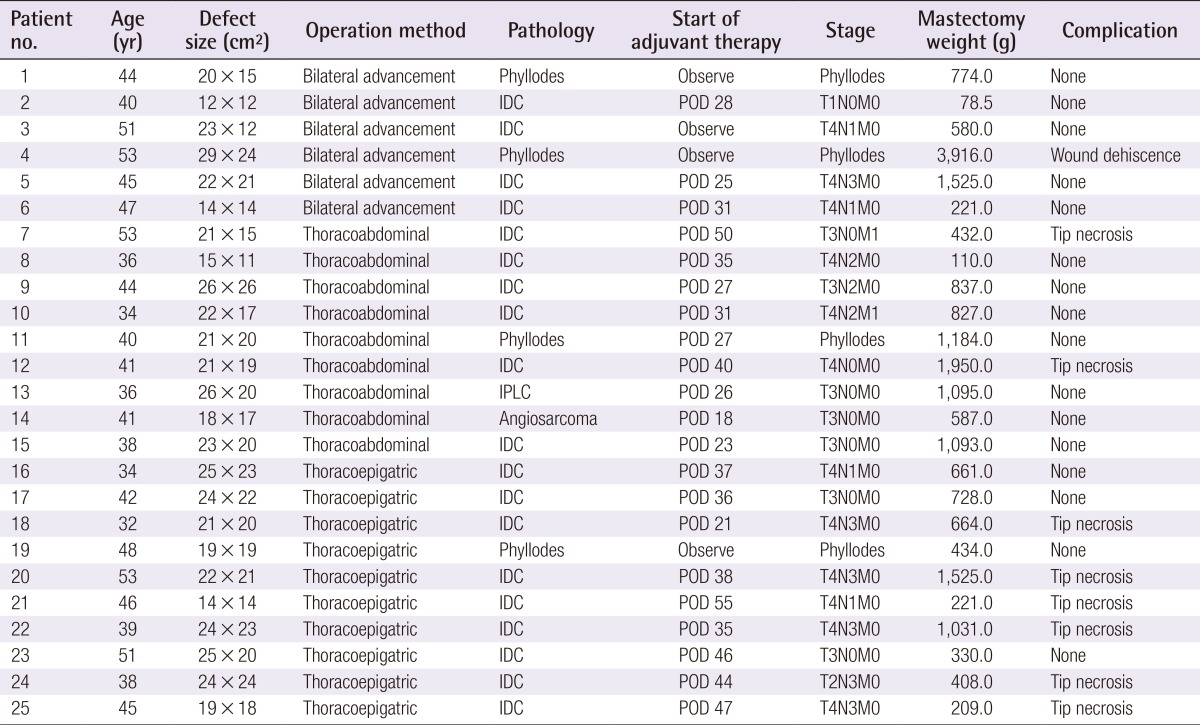
Among 25 local flaps in 24 patients, 6 were bilateral advancement (BA) flaps, 9 were thoracoabdominal (TA) flaps, and 10 were thoracoepigastric (TE) flaps. Clinical outcomes were compared including complications, the need for a secondary surgical intervention, and the timing of adjuvant therapy.
Results
The mean defect size was 436.2 cm2. Two patients with TA flaps and 6 patients with TE flaps developed distal flap necrosis, and skin grafts were needed to treat 2 patients with TE flaps. Radiation was administered to the BA, TA, and TE patients after average postoperative durations of 28, 30, or 41 days, respectively. The incidence of flap necrosis tended to be higher in TE patients, which lead to significant delays in adjuvant radiation therapy (P=0.02).
Conclusions
Three types of local skin flaps can be used to treat large chest wall defects after the excision of locally advanced breast cancer. Each flap has its own merits and demerits, and selecting flaps should be based on strict indications based on the dimensions and locations of the defects.
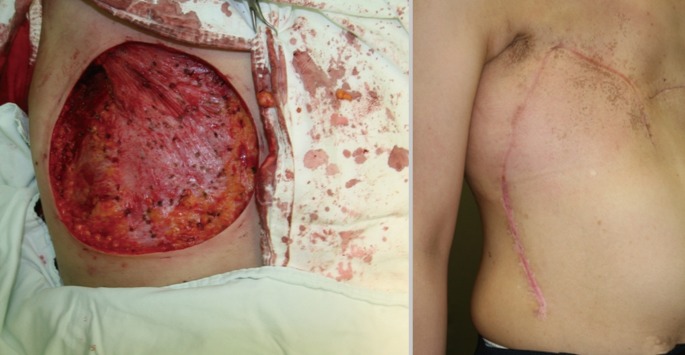
INTRODUCTION
Surgical ablation of locally advanced breast cancer often results in huge defects, however immediate reconstruction of the breast mound is controversial, particularly its relationship to clinical indications and type of reconstruction. Adequately covering any large chest wall defect is the main clinical issue, and a variety of techniques have been implemented over the last four decades, including skin grafts, local skin or fasciocutaneous flaps, omental flaps, and myocutaneous flaps such as pectoralis major, rectus abdominis, latissimus dorsi, and external oblique flaps [1,2,3,4,5,6,7,8]. Generally, flaps are advantageous over skin grafts in terms of aesthetics and durability (Fig. 1), especially when adjuvant radiation therapy is indicated [1,9]. Skin flaps are usually preferred to myocutaneous flaps because of their relative simplicity and comparable results [2,9]. We have used three types of skin flaps to cover large soft tissue defects after the surgical ablation of locally advanced breast cancer. The purpose of this study was to detail our experiences using bilateral advancement (BA), thoracoabdominal (TA), and thoracoepigastric (TE) flaps with a specific focus on outcomes, advantages, disadvantages, and proper patient selection.
METHODS
All mastectomies that required immediate reconstruction by a plastic surgeon at a single center between June 2008 and October 2013 were retrospectively reviewed. Breast mound reconstructions that used flaps and/or implants were excluded. Forty-three patients (45 breasts) were referred to plastic surgeons during the study period to receive chest wall reconstructions because of failed direct wound closure after mastectomy. Of these cases, 14 breasts received split-thickness skin grafts and 6 breasts received full-thickness grafts. A total of 25 local flaps were performed on 24 patients: 6 BA flaps, 9 TA flaps, and 10 TE flaps (a flap and a skin graft were performed on each breast in 1 patient) (Table 1). Chart review was performed to obtain data on sex, age, diagnosis, oncological status, adjuvant therapy, location and size of the defects, and complications. Outcomes were compared between all three groups based on the flap type. Due to small number of patients in each group, statistical comparisons were only performed to assess the overall incidence of complications and duration before the initiation of the adjuvant therapy. Analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA).
Surgical techniques
BA flap
For the BA flap, sufficient dissection begins at the margins of the mastectomy defect and progresses upward over the clavicle and downward almost to the level of the umbilicus without additional incisions. The plane of dissection is prefascial, and perforators from the epigastric and intercostal vessels are preserved whenever possible. The created cephalic and caudal flaps are sutured together, leaving a horizontal scar (Fig. 2). Trimming of "the dog ear" is usually necessary. This flap is indicated when the vertical dimensions of the flap do not exceed approximately 15 cm and its shape is approximately elliptical. If excessive tension develops during closure, a TA or TE flap should be considered.
TA flap
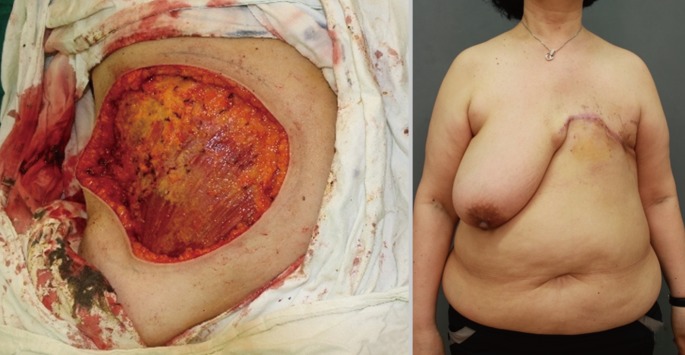
The TA flap is basically a rotation-advancement flap that uses the lateral intercostals, subcostal, and lumbar arteries. An incision is made at the midline of the abdomen all the way down to the umbilicus, and further dissection proceeds inferiorly and laterally across a prefascial plane. The pedicle of this flap can be identified at the medial edge of the external oblique muscle and preserved. The flap is rotated clockwise for left chest wall defects, or counterclockwise for right chest wall defects (Fig. 3). This flap is usually indicated when a higher portion of the defect lies medial, or a large amount of medial advancement is required.
TE flap
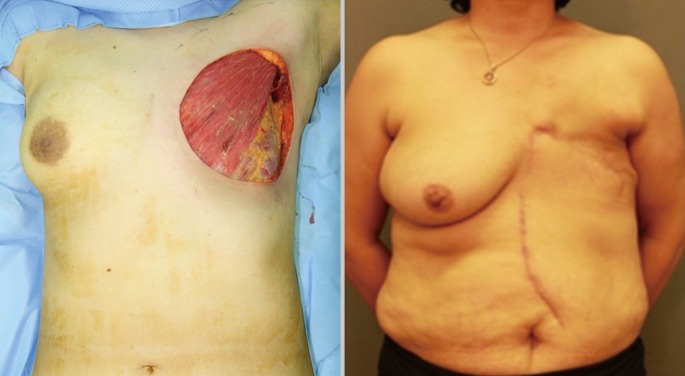
The TE flap is like a mirror image of the TA flap and uses perforators from the superior epigastric artery. The incision starts at the lower lateral angle of the defect and curves laterally down along the midaxillary line. Dissection continues medially and inferiorly, thereby preserving the superior epigastric perforators that pierce the rectus abdominis fascia (Fig. 4). This flap is usually indicated when the required medial advancement is relatively small and a higher portion of the defect lies laterally toward the axilla.
RESULTS
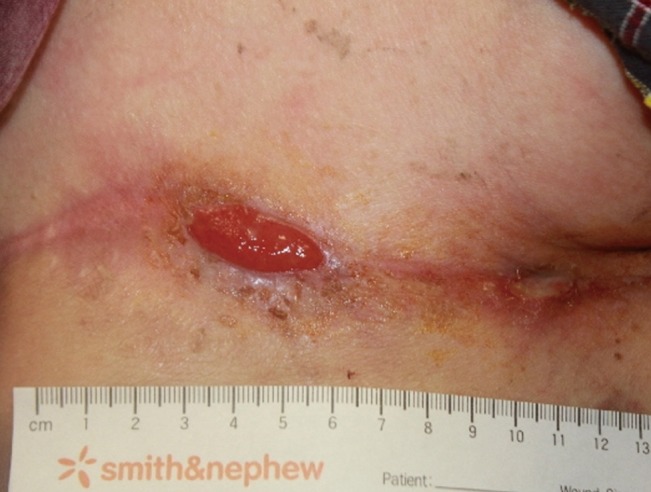
Between June 2008 and October of 2013, 25 local flaps were performed on 24 patients to cover chest wall defects after surgical ablation for locally advanced breast cancer. Among 24 patients, 23 were female and the mean age was 42.6 years (range, 32-53 years). Nineteen patients were diagnosed with invasive ductal carcinoma of the breast, 4 with an invasive phyllodes tumor, and 1 with a bilateral recalcitrant invasive phyllodes tumor. The mean follow-up period was 14 months (range, 4-66 years). The mean specimen weight was 1,382.5 g (range, 110-7,500 g; median, 894.5 g). The mean defect size was 400.1 cm2 (range, 90-696 cm2): 321 cm2 in BA flap group (n=6) vs. 462 cm2 in TA flap group (n=9) vs. 391 cm2 in TE flap group (n=10). In With the bilateral advancement flap, the cephalic and caudal flaps are elevated and sutured together, which leaves a horizontal scar. Fig. 2. Bilateral advancement flap after mastectomy total, 9 complications were recorded (36% of patients): 1 case of wound dehiscence (16.6%) in the BA flap group; 2 cases (22%) of distal flap necrosis in the TA flap group; and 6 cases (60%) of distal flap necrosis in TE flap group (P=0.17; Fisher exact test). All complications (except in 3 patients in the TE group) spontaneously healed in less than 3 weeks with conservative wound management (Fig. 5). Among the 6 patients with distal flap necrosis in the TE flap group, 1 patient underwent surgical debridement to promote wound healing and 2 patients eventually required skin grafts before initiating adjuvant radiation. Adjuvant chemotherapy and/or radiation were indicated for 3, 9, and 9 patients in the BA, TA, and TE groups, respectively. Adjuvant therapy commenced when sufficient wound healing was confirmed by the plastic surgeons. Adjuvant therapy was initiated after an average of 28, 30.1, or 41.4 postoperative days in BA, TA, and TE groups, respectively, and this difference was statistically significant between the TA and TE groups (P=0.02).
DISCUSSION
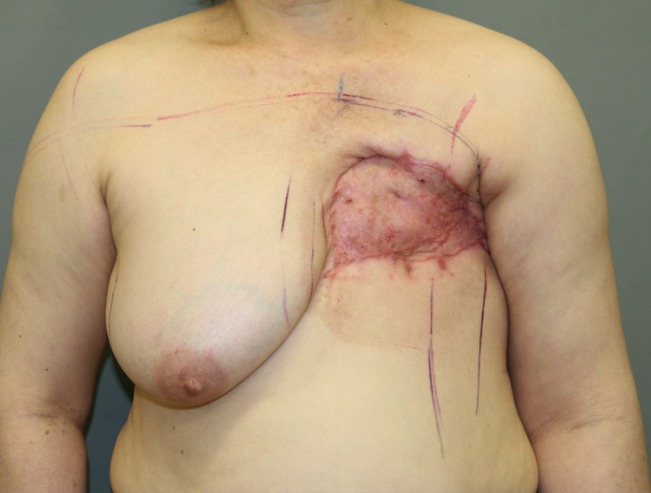
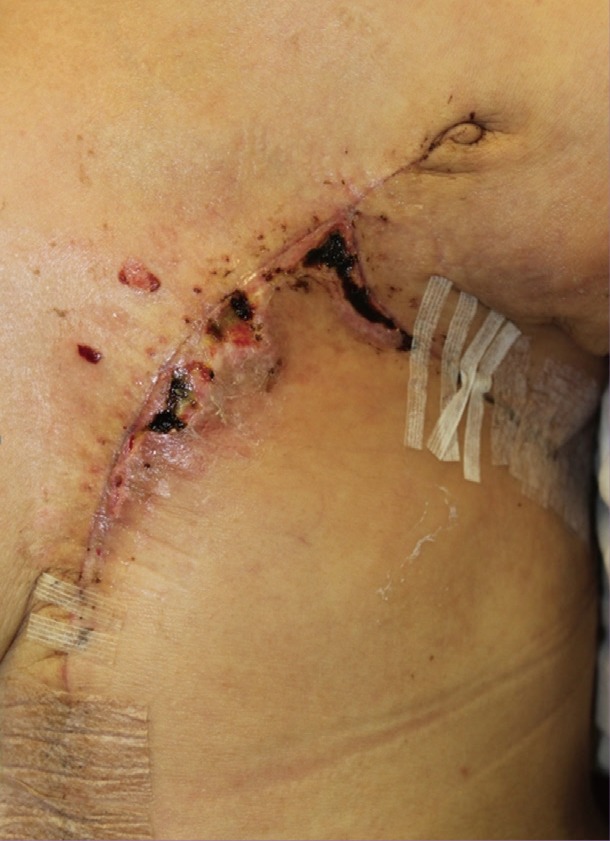
In contrast to the dramatic evolution in the field of breast reconstruction, less attention has been paid to reconstructing large chest wall defects following a so-called "toilet mastectomy", wherein the mastectomy is performed on locally advanced breast cancer patients with the aim of ablating the breast and skin tissues and minimizing oncologic recurrence [10,11]. A variety of locoregional tissue transfers have been introduced, and the common goal is to provide early wound healing and a low risk of total flap failure [1,2,3,4,5,8,9,12,13]. The BA flap is a straightforward way of closing the wound that only leaves a horizontal scar in the chest wall [4]. However, defects with greater vertical dimensions cannot be covered using this technique, and tension may result in wound dehiscence: as was the case here when the vertical dimension of the defect was 19 cm (Fig. 6).
Wound dehiscence in a bilateral advancement flap
Excessive tension during closure of the bilateral advancement flap may result in delayed healing or wound dehiscence.
The TA flap uses the skin, subcutaneous tissue of the anterior abdominal wall, and the direct perforating vessels of the segmental arteries that arise from the subcostal, intercostals, and lumbar arteries [2,3]. Epigastric perforators can also be preserved whenever possible. Deo et al. [2] reported that this flap is better than the myocutaneous flap in terms of mean blood loss, operating time, and length of hospital stay, and Persichetti et al. [3] have described using extended TA flaps to repair extensive defects ≤600 cm2. We treated 2 patients (22%) with small distal flap tip necrosis, and both spontaneously healed within 3 weeks. We believe perfusion to the TA flap is relatively robust, but the main drawback of this flap is the vertical midline scar.
The TE flap is mainly supplied by perforators from the superior epigastric arteries [1,5,6]. This flap has been confused with the TA flap because of its similar nomenclature: the two terms have been called a "medially based TA flap" or "laterally based TE flap" [1,5]. The TE flap is traditionally a transversely designed transposition flap supplied by the superior epigastric artery, which was previously used to repair upper extremity defects [14]. When transversely designed, the donor site often requires skin grafts or surgery on the opposite side of the abdomen [1,5,6]. Using a midaxillary incision, the vertical scar can be concealed when the arm is in a neutral position. Our large rotation-advancement flap design has the additional advantage in that the majority of the flap can be reelevated and reused by the time scarring occurs. However, this flap is hemodynamically weak, as reported by Baroudi et al. [15], and behaves more like a random flap.
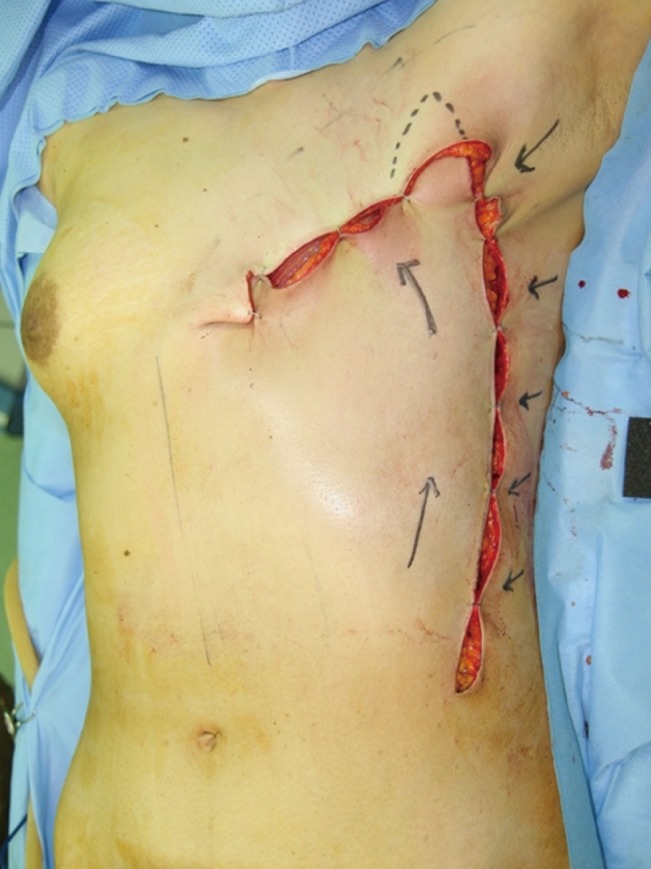
Here, 60% of the patients in the TE flap group developed distal flap necrosis. Among these, 2 patients required skin grafts because healing was delayed for more than 3 weeks due to significant necrosis. We do not know the exact reasons for this high incidence of flap-tip necrosis, although the problem could be due to the design or innate blood supply to the flap. However, considering the perforator theory, our design transfers the exact same anatomic region as the transverse or oblique design and uses the same perforator. The distal portion of this flap usually goes around the axillary area, where some redundancy in the local tissues allows relatively less closing tension (Fig. 7). So, we believe the cause of tip necrosis in the TE flap is its innate vulnerability to perfusion despite the inclusion of the superior epigastric perforators, especially when the defect extends too far laterally. We recommend debridement and skin grafting when perfusion in the distal part of the flap is suspected during the primary operation (Fig. 8).
Closing the thoracoepigastric flap
Closing the distal part of a thoracoepigastric flap is reduced due to some redundancy in the axillary region. Hence, distal necrosis in the thoracoepigastric flap could be attributed to an innate vulnerability to perfusion.
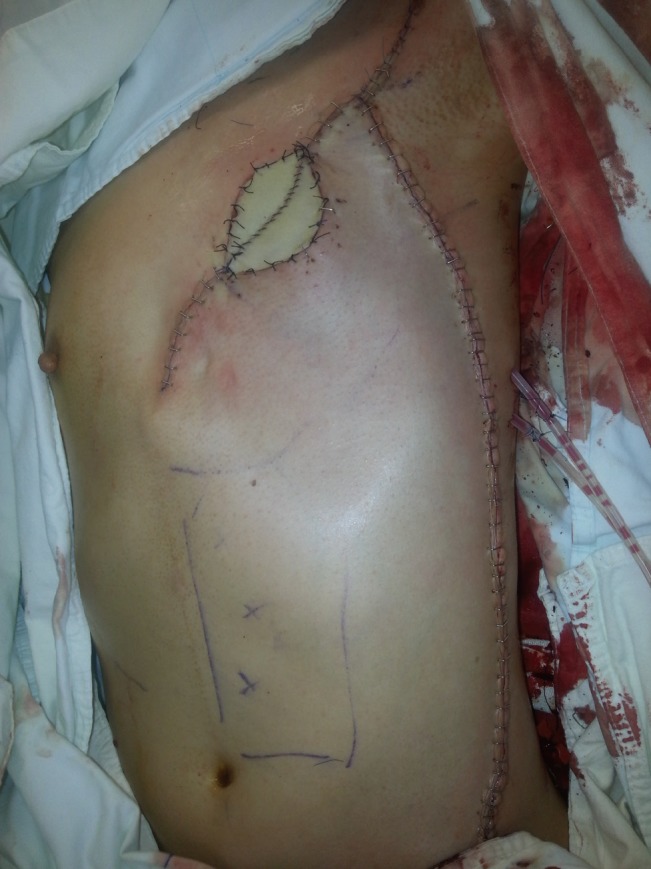
Managing distal-tip necrosis in the thoracoepigastric flap
Primary debridement and skin grafting is recommended when perfusion in the distal portion of a thoracoepigastric flap is suspected after flap transposition.
Oncological safety is the primary concern in patients with locally advanced breast cancer, with notable advances now made in multimodality anticancer therapy, but patients who receive mastectomy followed by chest wall reconstruction using grafts or flaps may want to delay the breast reconstruction [16]. Therefore, the primary donor sites for subsequent reconstructions should be preserved whenever possible, such as the lower abdominal tissues and/or latissimus dorsi, in order to maximize the final outcomes.
In summary, the three types of local skin flap described here could be applied to locally advanced breast cancer surgeries that leave a large chest wall defect. Each flap has its own advantages and disadvantages, and selection should be based on the dimensions and location of the defect.
Notes
This article was presented at the 2013 International Congress of the Korea Society of Plastic & Reconstructive Surgeons on November 1-3, 2013 in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.