Benign Lymphoepithelial Lesion of Parotid Gland and Secondary Amyloidosis as Concurrent Manifestations in Sjögren's Syndrome
Article information
Benign lymphoepithelial lesion (BLEL) is also known as lymphoepithelial sialadenitis, lymphoepithelial lesion, myoepithelial sialadenitis, and Sjögren's-type sialadenitis. It was first reported by Johann von Mikulicz-Radecki (1888) as synchronous inflammation of lacrimal and salivary glands [1]. Sjögren then referred to it as sicca syndrome, owing to salivary gland hyposecretion [1]. Goldwin first described the lymphoepithelial changes of BLEL in major salivary glands, presenting as unilateral or bilateral glandular enlargement without clinical signs of Sjögren's syndrome [1]. However, Morgan and Castleman were the first to detail the histopathologic hallmarks of BLEL, namely marked lymphoplasmacytic infiltration with residual epimyoepithelial islands (punctuated by hyperplastic and metaplastic ducts) and acinar degeneration [1].
In amyloidosis, insoluble polymeric protein fibrils are deposited in tissues and organs extracellularly. Secondary (AA) amyloidosis is characterized by accumulation of serum amyloid protein that is triggered in the setting of chronic inflammatory or infectious disease [2].
Reported here, with a review of the literature, is a patient with BLEL and secondary amyloidosis of the parotid gland as concurrent manifestations of Sjögren's syndrome.
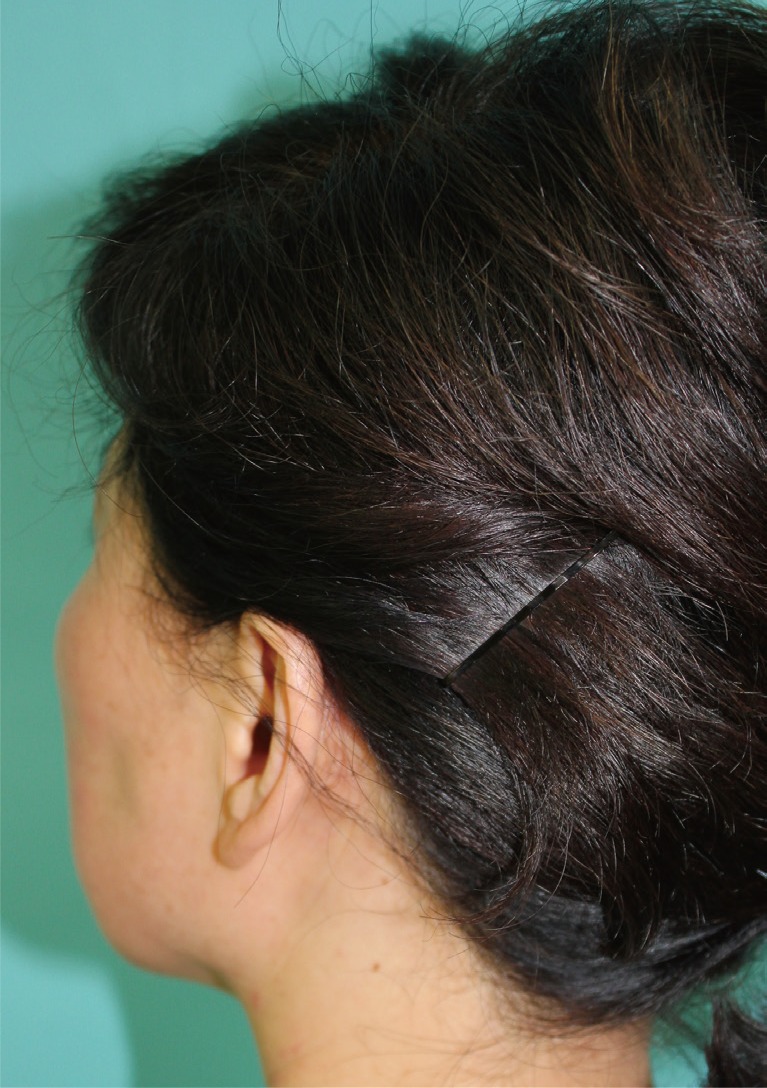
A 51-year-old patient arrived at our facility with a left cheek mass (3 cm in diameter), which was firm, fixed, and non-tender, although eliciting related complaints of tightness and mild pain (Fig. 1). Upon assessment of facial features, mild, bilateral enlargement of lacrimal and submandibular glands was evident, without palpable neck nodes. Computerized tomography (CT) of the neck (with contrast) showed diffuse swelling of both parotid glands, more so on left, where another ill-defined mass was present, raising suspicion of pleomorphic adenoma or carcinoma (Fig. 2). On this basis (and considering the duration of illness), conventional superficial parotidectomy was subsequently performed. Intraoperatively, the left-sided mass was near rupture, with dense periglandular adhesions and two enlarged nearby lymph nodes. Microscopic examination showed that ductal and acinar elements were heavily infiltrated by benign lymphoid cells, and discrete germinal centers were visible, all leading to a diagnosis of BLEL (Fig. 3A). Considerable parenchymal deposition of amorphous eosinophilic material was also seen (Fig. 3B). The latter displayed the characteristic apple-green birefringence of amyloid on Congo red stain.
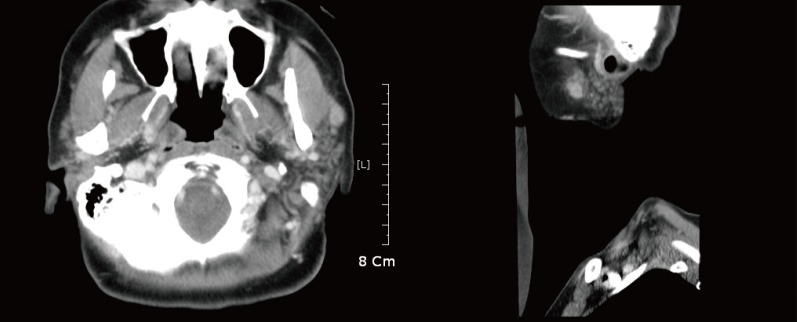
Contrast-enhanced computed tomography of neck: note bilateral diffuse swelling of parotid glands, more so on left; greater enhancement accentuates irregularly shaped 3-cm mass of left parotid gland, which proved to be amyloid.
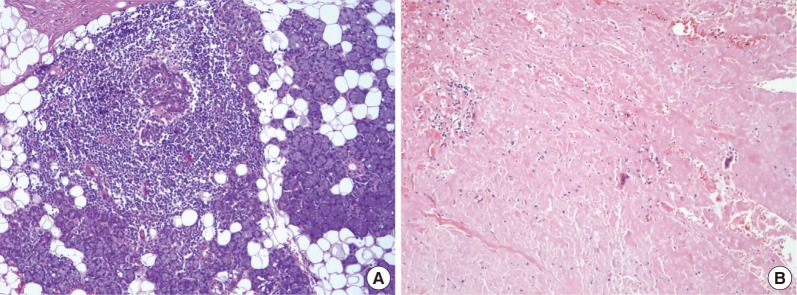
Microscopic views (H&E stain) of parotid gland mass: (A) obliteration of ducts and acini by benign lymphoid infiltrates forming germinal centers (200×); (B) considerable parenchymal deposition of amorphous eosinophilic material, later confirmed as amyloid on Congo red stain (200×).
Due to claims of mild eye and mouth dryness in the 3 months prior to this visit, diagnostics and treatment for potential Sjögren's syndrome were initiated postoperatively. Salivary gland scintigraphy showed very low radionuclide uptake by parotid and submandibular glands bilaterally (Fig. 4). Laboratory indices were positive for rheumatoid factor, anti-nuclear antibody, anti-Sjörgen's-syndrome-related antigen A (anti-SSA, anti-Ro), and anti-Sjörgen's-syndrome-related antigen B (anti-SSA, anti-La). Serum levels of immunoglobulin G (IgG) (2,220 mg/dL) and immunoglobulin A (IgA) (476 mg/dL) were elevated, but immunoglobulin M (IgM) (121 mg/dL) and IgG4 immunoglobulin fractions (87.9 mg/dL; reference range, 39.2-894.0 mg/dL) were within normal ranges. Results of Schirmer's test (left, 9 mm; right, 30 mm) and break-up time scores (left, 2 sec; right, 4 sec) were low. As suspected, the patient's profile fulfilled revised international classification criteria for Sjögren's syndrome [3].
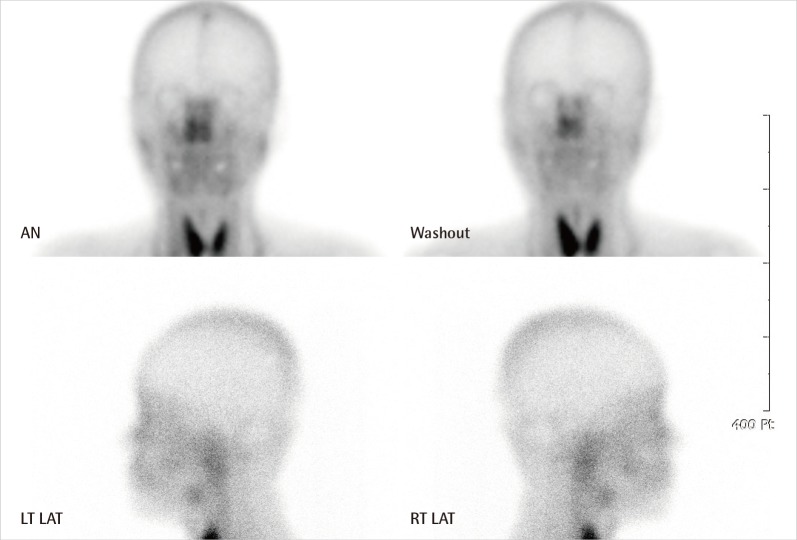
Scan of salivary glands: markedly diminished uptake of radionucleotide in both parotid and submandibular glands, relative to thyroid gland. AN, anterior; LT LAT, left lateral; RT, right.
Following surgery, the patient was given pilocarpine eye drops for treatment of dry eyes. Other than mild residual hollowing of the left cheek, she suffered no true complications during a one-year follow-up period, and she refused recommended monitoring (ultrasonography or computed tomography) to check for recurrence.
BLEL is considered an autoimmune disease of salivary glands. Clinical features of Sjögren's syndrome may be manifested by 50% to 84% of patients with BLEL, which increases the risk (40:1) of developing malignant lymphoma, especially in long-standing BLEL. Hence, follow-up surveillance for potential malignant transformation is advised [4].
Amyloid is seen as eosinophilic fibrils in hematoxylin and eosin (H&E)-stained tissue; and on Congo red stain, its regularly structured β-pleated sheets result in a unique apple-green birefringence under light microscopic polarization. AA amyloidosis is typically associated with chronic inflammatory disease and may be confirmed via immunohistochemistry [2].
In patients with salivary gland enlargement, diagnosis of BLEL may be difficult due to the many benign inflammatory conditions that arise and/or the occurrence of salivary cysts or tumors. For differential diagnostic purposes, imaging modalities such as CT, ultrasound, technetium scan, and sialography may be helpful, although such technical advancements are not routinely utilized in the management of salivary gland tumors. Fine needle aspiration biopsy is controversial in this setting, because for many surgeons, the presence of tumor alone is sufficient indication to operate. With a sensitivity near 90% and a specificity of 75%, fine needle aspiration (FNA) biopsy may have a role in documenting the benign disease of poor-risk patients, thus avoiding inappropriate surgery [5]. Nevertheless, histologic findings may be precarious. A history of autoimmune disease or complaints of dry eyes or mouth often are diagnostic clues, given a heterogeneous lymphoid component. Grounds for diagnosis of BLEL by FNA include the presence of epimyoepithelial islands, numerous small lymphocytes, and a granular proteinaceous background [5].
Concurrent BLEL and secondary amyloidosis affecting parotid gland unilaterally have not been reported to date. In this instance, the irregularly contoured margins of accumulated amyloid and the dense periglandular adhesions observed, both induced by inflammation, are easily interpreted radiographically and grossly as signs of pleomorphic adenoma or features of malignancy. Because BLEL may be a consequence of human immunodeficiency virus infection or may progress to Hodgkin's lymphoma, history-taking is critical, and lumpectomy may be beneficial [5]. Surgical removal of such tumors is often tempting to exclude malignancy, but various radiologic modalities and/or FNA biopsy do have merit in this context, utilizing a multidisciplinary approach (i.e., rheumatologist, pathologist, and ophthalmologist) to establish a diagnosis and determine appropriate treatment. In a patient with documented autoimmune disease, the differential diagnosis of a cheek mass should include benign systemic conditions. As seen here, focal deposition of amyloid in BLEL may mimic pleomorphic adenoma or malignancy of the parotid gland.
Notes
The authors thank Sung-Eun Kim, MD and Jae-Hyun Suk, MD, for kind assistance in clinical and pathological description of this article.
This article was presented as a poster at the 70th Congress of the Korean Society of Plastic and Reconstructive Surgeons on November 9, 2012 in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.