One Stage Reconstruction of Skull Exposed by Burn Injury Using a Tissue Expansion Technique
Article information
Abstract
Background
An area of the skull exposed by burn injury has been covered by various methods including local flap, skin graft, or free flap surgery. Each method has disadvantages, such as postoperative alopecia or donor site morbidities. Due to the risk of osteomyelitis in the injured skull during the expansion period, tissue expansion was excluded from primary reconstruction. However, successful primary reconstruction was possible in burned skull by tissue expansion.
Methods
From January 2000 to 2011, tissue expansion surgery was performed on 10 patients who had sustained electrical burn injuries. In the 3 initial cases, removal of the injured part of the skull and a bone graft was performed. In the latter 7 cases, the injured skull tissue was preserved and covered with a scalp flap directly to obtain natural bone healing and bone remodeling.
Results
The mean age of patients was 49.9±12.2 years, with 8 male and 2 female. The size of the burn wound was an average of 119.6±36.7 cm2. The mean expansion duration was 65.5±5.6 days, and the inflation volume was an average of 615±197.6 mL. Mean defect size was 122.2±34.9 cm2. The complications including infection, hematoma, and the exposure of the expander were observed in 4 cases. Nonetheless, only 1 case required revision.
Conclusions
Successful coverage was performed by tissue expansion surgery in burned skull primarily and no secondary reconstruction was needed. Although the risks of osteomyelitis during the expansion period were present, constant coverage of the injured skull and active wound treatment helped successful primary reconstruction of burned skull by tissue expansion.
INTRODUCTION
Burn wounds require different treatments depending on their location and severity. The scalp is the only area in the body with dense hair patterns and reconstructive options are therefore limited. Scalp wounds with calvarial exposure present a complex clinical challenge and require a systematic approach for reconstruction. Local flaps are ideal for small wounds but are rarely adequate for larger defects. Skin grafts can be a useful option, but require removal of the outer table, thus increasing the complexity and risks of the reconstruction. Skin grafts on trabecular bone also break down easily. A free flap is an option, but requires microvascular expertise and a complex, multi-staged reconstructive effort with the possibility of donor-site morbidity. Scalp-tissue expansion is a validated, reliable, and safe technique for the treatment of alopecia and has been commonly used in secondary reconstruction in skull injury [1-3]. In the acute stage, the wound is excised and temporarily closed with a skin graft or flap. Tissue expansion is initiated once there is stable coverage. Austad et al. [4] argued against tissue expansion in acute injuries because of the risk of contamination and implant exposure. However, tissue expansion was used successfully in our series for the primary reconstruction of burned skulls, with acceptable complications and good long-term results. This paper reports our 10 years of experience and a review of the literature.
METHODS
Study subjects
From January 2000 to 2011, 10 patients presented to the Hangang Sacred Heart Hospital with a burned scalp and an exposed skull after sustaining an electrical burn injury.
Surgical methods
Rectangular type expanders (Nagosil, Nagor Ltd., Glasgow, UK) were used. The injection port of the expander was replaced with a mini dome valve prior to use. The air within the expander was removed completely, and the four corners of the expander were folded gently to prevent perforation of the flaps by the expander. The horizontal and vertical sides of the space where the expander would be inserted were larger than the entire area by approximately 2 to 3 cm when compressed without sharply folding the four corners of the tissue expander. These sides were drawn in order to prevent the folding of the tissue expander during its insertion. Upon the insertion of the tissue expander, an incision was made at the boundary of the skull's injured area. The length was approximately half of the compressed tissue expander. Through this incision, a small pouch was made by subgaleal dissection; a silicone tube was placed by preparing a tunnel that penetrated the skin area away from the tissue expander. Hemostasis was performed on bleeding areas by electrocautery. The absence of bleeding was verified by washing with saline; a tissue expander was inserted into the prepared pouch. Prior to the insertion, leakage tests were performed using saline, and the air within the tissue expander was removed. After the insertion, the expander was inflated with 20 to 30 mL saline up to the volumein which the tissue expander unfolded, a drain was installed, and a two-layer suture was performed. After surgery, a Hemovac (Zimmer Inc., Warsaw, IN, USA) was used to prevent hematoma, and it was maintained from 5 to 7 days after surgery. Starting 2 weeks after the insertion, 5 to 10 mL saline was injected all at once, 4 to 5 times a week at our outpatient clinic for approximately 9 to 11 weeks while assessing the development of side effects (e.g., flares in the flap, paleness, necrosis). During the expansion period, constant skull coverage was maintained with foam dressing materials such as Mepilex (Mölnycke Health Care AB, Goteborg, Sweden) and an antibiotic ointment such as Bactroban (GlaxoSmithKline, Middlesex, United Kingdom) or Teramycin (PT Pfizer Inc., New York, NY, USA) to prevent skull desiccation or the development of osteomyelitis. In addition, frequent and active wound treatment was performed by wound dressing and irrigation with normal saline 3 to 4 times a week. Preventive antibiotics were prescribed during the expansion period. When the expansion reached the desired level, the tissue expander was removed. Granulation tissue and necrotic tissue were removed and the injured skull was resected or burred by a diamond burr (Asculap Inc., Tuttlingen, Germany). The affected area was covered with a displacement, advancement, or rotation scalp flap. In the initial period, after the removal of injured skull tissue, costal cartilage or adjacent bone tissue was divided into two and grafted to the skull defect. In 7 cases which were done recently, the injured skull was preserved and covered by a scalp flap directly. By preserving the injured skull and covering it with expanded vascular-rich scalp tissue, the burned skull was successfully primarily reconstructed in all 7 cases.
RESULTS
The sex of the patients, their age, injured sites, the type and number of tissue expanders used, the duration of the expansion period, expansion volume, surgery outcomes, complications, and the length of the follow-up period are shown in Table 1.
Out of 10 patients, 8 were male. The mean age of the patients was 49.9±12.2 years (Table 1). The temporal region was the most prevalent with 5 cases, followed by the occipital area in 3 cases, the frontal area in 1 case, and the parietal area in 1 case. The average defect size was 119.6±36.7 cm2. The number of tissue expanders applied to 10 patients was 13. The expansion period was between 63 days and 80 days; the average was 65.5±5.6 days, depending on the location and size of the lesion. The total volume of saline injected into the tissue expanders was from 345 to 925 mL, and the average was 615±197.6 mL. An average of 8% of volume was over-expanded in comparison of expanders. In 7 cases, 1 tissue expander was used for reconstruction. For 3 cases, 2 tissue expanders were used. Complications were observed in 4 cases. Infection, which was caused by osteomyelitis that developed in the injured bone, occurred in 2 cases: hematoma in 1 case and exposure of the expander due to skin necrosis during expansion in another case. Among the 4 cases with complications, secondary revision was needed in 1 case in which osteomyelitis was not cured after 3 additional months of treatment and a bone debridement operation was subsequently performed. After revision, the wound was cured and no recurrence was observed in 1 to 10 years of follow-up care (Table 1).
CASES
Case 1
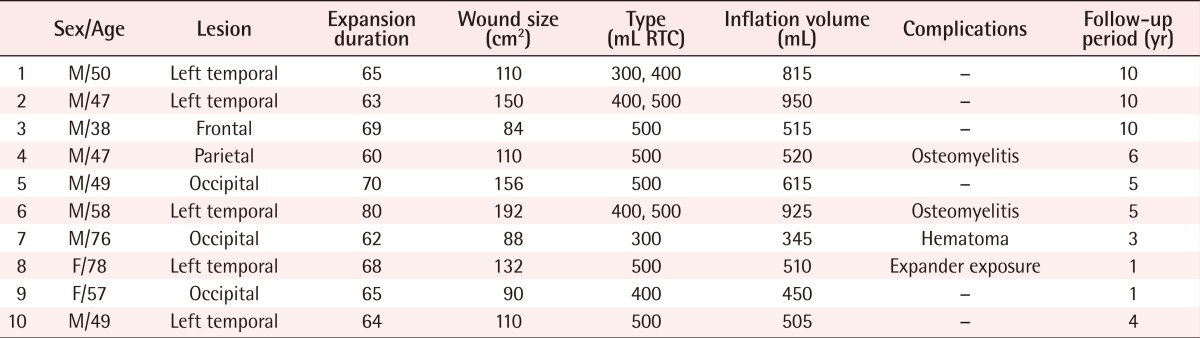
A 47-year-old male patient was admitted due to a scalp injury (10×15 cm) caused by an electric burn. Two tissue expanders (400 mL and 500 mL) were inserted. For 63 days, saline (up to 950 mL) was injected, while necrotic soft tissue and necrotic bone tissue were removed. A bone graft was performed by using a bihalved rib, followed by covering the area with an expanded flap by advancing and rotating. In the 3D CT performed 1 year after surgery, well-engrafted bones were detected. In the follow-up observation 10 years after surgery, satisfactory results were observed in the volume and direction of hair growth (Fig. 1).
Tissue expansion with bone graft
(A) Full expanded state of two expanders. (B) Harvested and bihalved rib bone. (C) Debridement including dead cortical bone. (D) Rib graft to skull defect. (E) Immediately after expander removal and local flap operation. (F) 3D CT image postoperative 1 year. The grafted bone is visibly viable. (G) After 10 years of follow-up. Good hair volume and direction.
Case 2
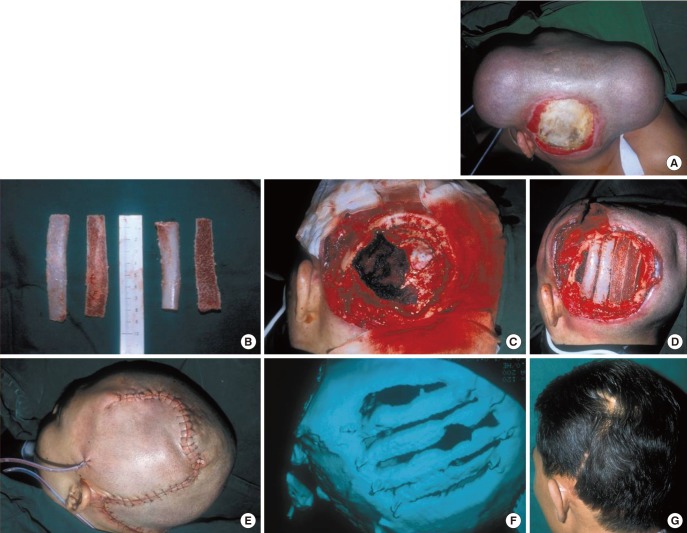
A 49-year-old male patient sustained a scalp injury (13×12 cm) caused by an electric burn. An incision was made along the parietal area of the scalp injury where 1 tissue expander (500 mL) was inserted. After surgery, 615 mL of saline was injected for 70 days. After the removal of necrotic tissues, injured bone was covered by advancing a scalp flap. One year after surgery the scalp showed natural bone healing and remodeling. After surgery, osteomyelitis was not observed during a 5-year follow-up period (Fig. 2).
Tissue expansion without bone graft
(A) Before operation, 13×12 cm skull defect. (B) After one tissue expander insertion. (C) After full expansion. (D) Immediately after expander removal and local flap. (E) Bone scan postoperative 1 year. No evidence of osteomyelitis. (F) After 5 years follow-up. Good hair volume and direction.
DISCUSSION
Numerous methods have been introduced for the reconstruction of the burned skull. For small lesions (<3 cm), primary closure can be effected [5,6]. Split-thickness skin grafts can be combined with a staged procedure, such as tissue expansion, to provide temporary wound coverage. The primary advantage of split-thickness skin grafting for scalp reconstruction is its technical simplicity. The disadvantage is the cosmetically inferior appearance of the skin graft compared with a scalp reconstructed with hair-bearing tissue. In addition, the long-term stability of coverage tends to be a problem. Local tissue arrangement including transposition, advancement, and rotation flaps have been employed in scalp reconstruction. Orticochea [7] published his four-flap scalp reconstructive technique by which defects as large as 30% of the cranium can be closed. The advantages of scalp flaps include excellent cosmesis, replacement of hair-bearing defects with hair-bearing scalp, a single donor site, and the technical simplicity of the procedure. Potential drawbacks include the relative lack of scalp mobility and the potential to displace mobile structures, such as the eyebrow or hairline. Tissue expansion is particularly useful in scalp reconstruction because it provides the surgeon with the opportunity to replace missing tissue with a tissue of similar quality and thickness [8-10]. The technique increases the amount of locally available tissue, preserves sensation, and maintains hair follicles and adnexal structures. In addition, tissue expansion produces a delay phenomenon, increasing the vascularity of the expanded flap [4]. Defects up to 50% of the scalp can be reconstructed without a noticeable change in hair density. Many authors felt afraid to place a tissue expander next to an open wound because it could predispose it to infection and may pull on the wound margins, thus enlarging the wound and risking prosthetic exposure [11,12]. The unique anatomy of the scalp, however, allows for safe and expeditious expansion despite these risks. The blood supply of the scalp is robust, hence decreasing the chances of infection. The tissue expanders placed under the scalp expand only the tissues directly above them. Perhaps because of the unyielding galea aponeurotica, the scalp about 1 to 2 cm away from the expander margin is generally undisturbed by tissue expansion. As a result, proximate placement of multiple tissue expanders can be well tolerated despite open wounds. This allows for the use of expansion, which is the most favorable reconstructive technique for the scalp, as it provides durable hair-bearing tissue [13]. However, one of the major disadvantages of tissue expansion is the length of time it takes to expand the adjacent scalp. Expansion periods often take 2 to 3 month and the risk of osteomyelitis of the exposed skull is increased during that period. For its prevention, the exposed skull was constantly covered with foam dressing materials and antibiotic ointment to maintain a moist environment. Wounds were treated and irrigated every other day. Appropriate antibiotics were intravenously administered during the expansion period. By these efforts, preservation of the damaged skull and coverage with expanded scalp tissue was successful. Oishi and Luce [14] have also recommended preservation of the bone and coverage with well-vascularized tissue when the skull is not grossly necrotic. Osteomyelitis was shown in 2 of the 10 cases. One patient was completely cured by wound dressing and continuous injection of antibiotics within approximately 3 months. Another patient was completely cured by revision of bone curettage. Afterwards, the osteomyelitis was completely cured and no recurrence was observed in 2 to 5 years of follow-up care. For the treatment of the injured skull in the initial period, bone grafting was performed immediately after the removal of the injured parts. In the latter period, the bone frame was maintained by preserving the injured bones. Natural healing and bone remodeling were induced by covering the injury with a flap with sufficient blood flow. The procedure was successful. With regard to the histological findings observed after the expansion of the skin by applying tissue expanders, the thickness of the epidermis was maintained [15-18]. On the other hand, the thickness of the dermis was decreased. Collagen synthesis was increased in the dermal layer, which also showed myofibroblasts. Adipocytes became thin and the thickness of the muscles was reduced. The size and number of dermal blood vessels were increased in the order of the capillaries, veins, and arteries, from least to most extensive increase. Thus, the blood supply in the expanded skin was increased relative to pre-expansion. It was concluded that flaps with such increased blood supply play an important role in bone remodeling and in the prevention and treatment of osteomyelitis. In addition, to minimize the development and progression of osteomyelitis, rapid tissue expansion was induced. The entire tissue expansion procedure was completed in between 63 days and 80 days. Rapid tissue expansion was performed by injecting 5 to 10 mL of saline at a time, 4 to 5 times a week. At each expansion procedure, flares in the stretched scalp tissues and changes in skin thickness were examined carefully. Thus, exposure of the tissue expander during the expansion did not occur except in 1 case, in which the patient developed skin necrosis. Even in this case, the exposure of the expander occurred after the substantial progression of the tissue expansion procedure; hence, normal tissue expansion could be completed.
Most third or fourth degree burns that could induce skull injury are primarily electric burns caused by a current higher than 22,000 volts. Even in an electric burn injury, the extremities such as the hand and foot are the areas most commonly injured; cases with skull injury are rare. Hence, during the 10-year follow-up observation period, only 10 cases could be examined. Thus, it is necessary to obtain more clinical research results by securing more cases.
Reconstruction was successfully performed on 10 patients with burned skulls by using a tissue expander in the acute stage. Even in cases of an exposed skull and when the occurrence of osteomyelitis was suspected, by combining aggressive treatment and the prevention of osteomyelitis with tissue expansion surgery, the burned skull could be reconstructed successfully. During that time, efforts should be made to rapidly expand tissue and treat the wound. Through such efforts, the burned skull could be successfully reconstructed by a one-stage tissue expansion surgery with acceptable complications and a good long-term outcome.
Notes
No potential conflict of interest relevant to this article was reported.