Outcome of Management of Local Recurrence after Immediate Transverse Rectus Abdominis Myocutaneous Flap Breast Reconstruction
Article information
Abstract
Background
No consensus has been reached regarding the outcome of management of local recurrence after transverse rectus abdominis myocutaneous (TRAM) flap breast reconstruction. This study demonstrated the presentation, management, and outcomes of local recurrence after immediate TRAM breast reconstruction.
Methods
A comparison was conducted among 1,000 consecutive patients who underwent immediate breast reconstruction with a pedicled TRAM flap (TRAM group) and 3,183 consecutive patients who underwent only modified radical mastectomy without reconstruction (MRM group) from January 2001 to December 2009. The presentation, treatment, and outcome including aesthetics and overall survival rate were analyzed.
Results
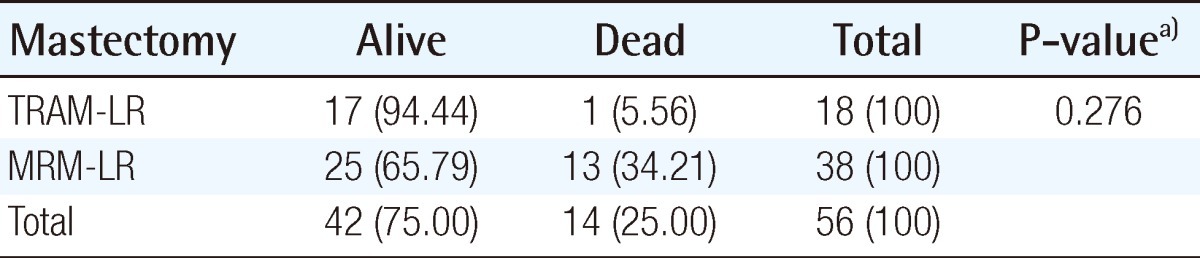
Local recurrences occurred in 18 (1.8%) patients (TRAM-LR group) who underwent TRAM breast reconstruction and 38 (1.2%) patients (MRM-LR group) who underwent MRM only (P=0.1712). Wide excision was indicated in almost all the local recurrence cases. Skin graft was required in 4 patients in the MRM-LR group, whereas only one patient required a skin graft to preserve the mound shape in the TRAM-LR group. The breast mound was maintained in all 17 patients that survived in the TRAM-LR group even after wide excision. The overall survival rate was 94.4% in the TRAM-LR group and 65.8% in the MRM-LR group (P=0.276).
Conclusions
Local recurrence after immediate TRAM flap breast reconstruction could be detected without delay and managed effectively by multiple modalities without reducing overall survival rates. Breast mound reconstruction with soft autologous tissue allowed for primary closure in most of the cases. In all of the patients who survived, the contour of their reconstructed breast remained.
INTRODUCTION
An immediate breast reconstruction after mastectomy has several aesthetic and psychological advantages [1]. Moreover, considering the fact that the peak age of developing breast cancer in Korea is the mid-forties, a patient's quality of life after surgery has added significance [2]. Pedicled transverse rectus abdominis musculocutaneous (TRAM) flap breast reconstruction has the undeniable benefits of long-term predictability and relative simplicity; hence, it remains the primary reconstructive modality worldwide [3]. Recently, the oncologic safety of immediate TRAM following skin-sparing mastectomy or nipple areolar skin-sparing mastectomy has been published [4,5]. Nevertheless, local recurrence after mastectomy is an unwanted event that is not always predictable. The authors present here the presentation, management, and outcome of local recurrence after immediate TRAM flap breast reconstruction.
METHODS
The medical information of all the patients who underwent immediate TRAM breast reconstruction by the senior author was recorded in a prospective database. Between January 2001 and December 2009, the medical records of 1,000 consecutive patients who underwent immediate breast reconstruction using pedicled TRAM flap were prospectively registered (TRAM group). The follow-up period was defined as from the first reconstructive date to the last outpatient date. In case of a patient with local recurrence or one who died, the follow-up period was defined from the first reconstructive date to the event date. Patients who dropped out during the study period were excluded from the study. The preoperative records included demographic factors, general medical status, and social habits. Data from the surgery such as the specific surgical technique and the weight of the mastectomy specimen were added to the database. Oncologic data such as the cancer stage and treatment, along with the postoperative clinical course including the complications, recurrence and metastasis, and overall outcome were added during the postoperative visits at the outpatient clinic.
No patients who underwent TRAM breast reconstruction had stage 4 cancer. For the purpose of comparison, the data of the patients who underwent modified radical mastectomy (MRM) without reconstruction in the same period were collected. After the patients with preoperative clinical stage 4 cancer were excluded, 3,183 patients remained (MRM group). Oncologic follow-up included clinical examinations, mammography, and chest X-ray every six months. Tomography, bone scan, ultrasonography, and other investigations were performed whenever deemed necessary by the oncologic surgeon.
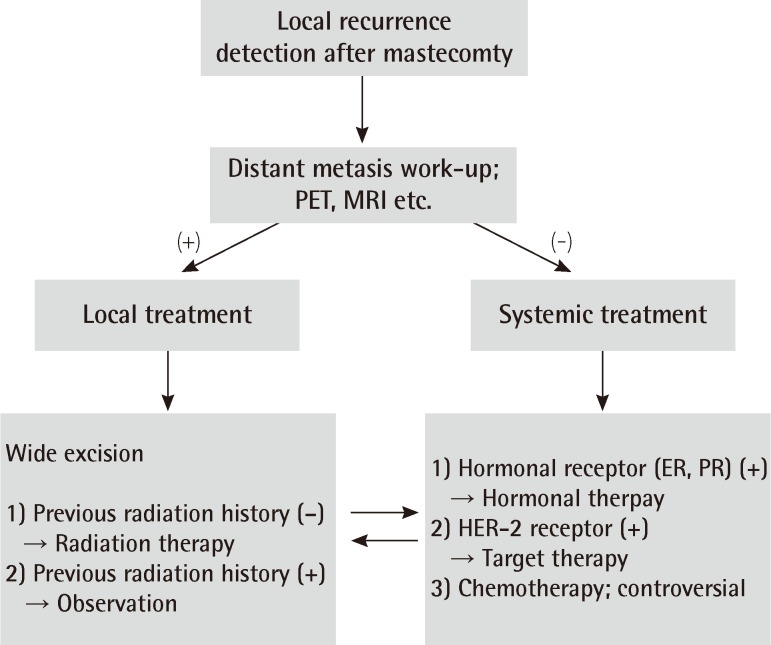
For the basic comparisons data such as the patients' age, follow-up period, cancer stage distribution, and the overall survival rate were collected from both the TRAM group and MRM group; the data were compared using the chi-squared test, Fisher's exact test, Mann-Whitney test, and log-rank test. We categorized the local recurrence rates and overall survival rates into a lower stage group (stage 0, 1) and higher stage group (stage 2, 3) in order to exclude the major confounding factor (stage). After local recurrence was diagnosed, the patient was further classified into the TRAM-local recurrence (LR) group or MRM-LR group. The management of local recurrence was demonstrated in Fig. 1. The overall outcome of each reconstructed breast (i.e., whether the shape of the reconstructed breast mound was well maintained thereafter) was evaluated by two plastic surgeons, including the senior author.
RESULTS
With a median follow-up of 57 months, 18 out of the 1,000 TRAM group patients and 38 out of 3,183 the MRM group patients experienced local recurrence.
Patients' demographics and oncologic profile
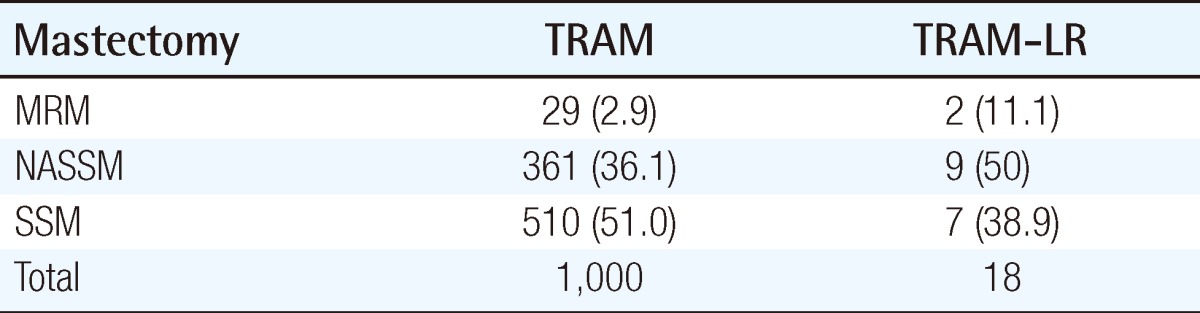
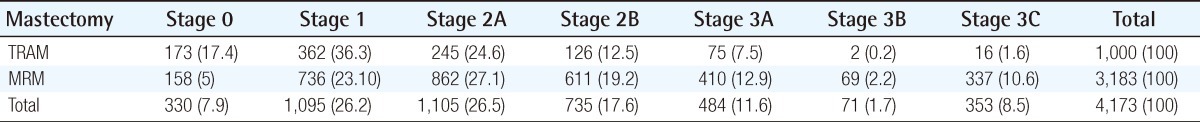
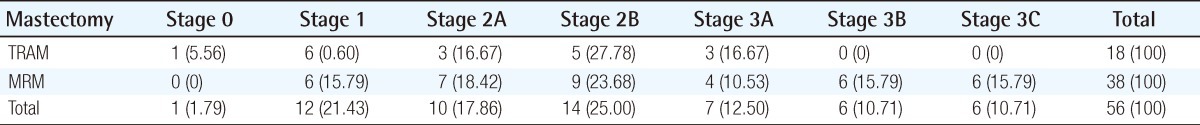
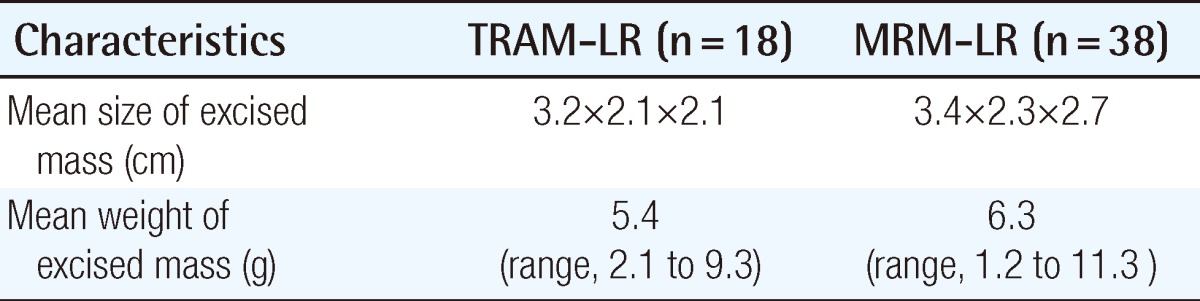
The mean age at the time of surgery was 42.2 years (range, 22 to 68 years) in the TRAM group and 47.9 years (range, 22 to 79 years) the in MRM group. The mean follow-up length in the TRAM group was 56.4 months (3 to 93 months) and 60 months (2 to 96 months) in the MRM group. The mastectomy method is demonstrated in Table 1. The stage distribution of the two groups is shown in Table 2. Stage distribution in the TRAM group tended to be lower than the MRM group.
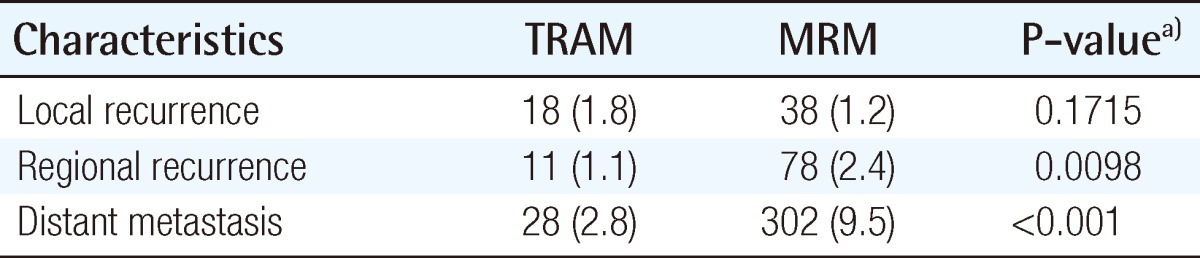
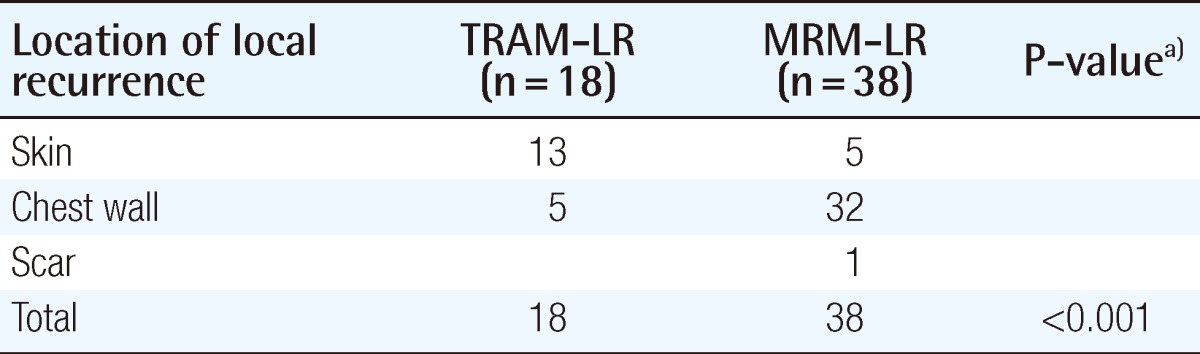
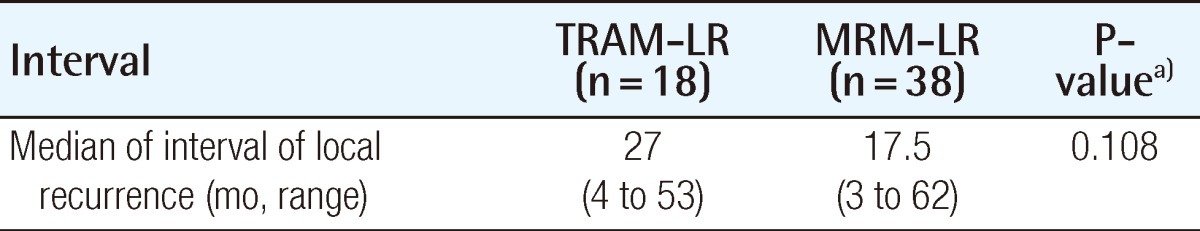
The recurrence or metastasis was classified as local, regional, or distant; its incidence is summarized in Table 3. The incidence of local recurrence was 1.8% in the TRAM group and 1.2% in the MRM group (P=0.1712). In both groups, the local recurrence ratestended to be higher in the higher stage of breast cancer (Table 4). In order to rule out the confounding effect of stage, we analyzed the local recurrence rates by classifying cases into a lower stage group (stage 0, 1) and higher stage group (stage 2, 3) (Fig. 2). The location of local recurrence along with the interval between the time of surgery and the detection of the local recurrence are demonstrated in Tables 5, 6. The most common site of local recurrence in the TRAM-LR group and MRM-LR group were the skin and chest wall, respectively. There was no significant difference in detection of the interval between the two groups (Table 6).
Local recurrence rate according to the stage
(A) Local recurrence rate in the lower stage group. (B) Local recurrence rate in the higher stage group. MRM, modified radical mastectomy; TRAM, transverse rectus abdominis myocutaneous.
Management of local recurrence, overall survival rate
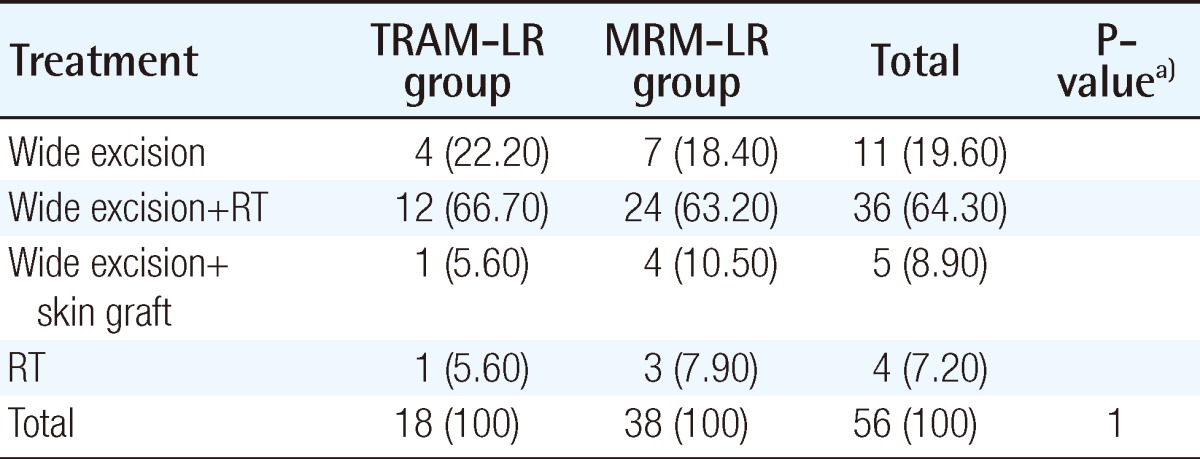
Treatment modalities for both groups are summarized in Table 7. Wide excision with or without radiation was required in 17 out of 18 patients in the TRAM-LR group and 35 out of 38 in the MRM-LR group. Wide excision with a safety margin of at least 1 cm in an elliptical pattern was the standard surgical procedure; this was performed by an oncologic surgeon (general surgeon). Frozen biopsy confirmed negative margins. If primary closure was impossible, a skin graft was performed by a plastic surgeon. A skin graft was required in 1 patient (5.9%) in the TRAM-LR group and 4 (11.4%) in the MRM-LR group. However, needing a skin graft failed to show a statistically significant difference between the two groups. Only one patient who developed local recurrence in the form of periareolar Paget's diseaseat 3.5cm in diameter needed a skin graft in the TRAM-LR group.
All of the breast mounds in the TRAM-LR group including the patient who underwent the skin graft were well maintained.
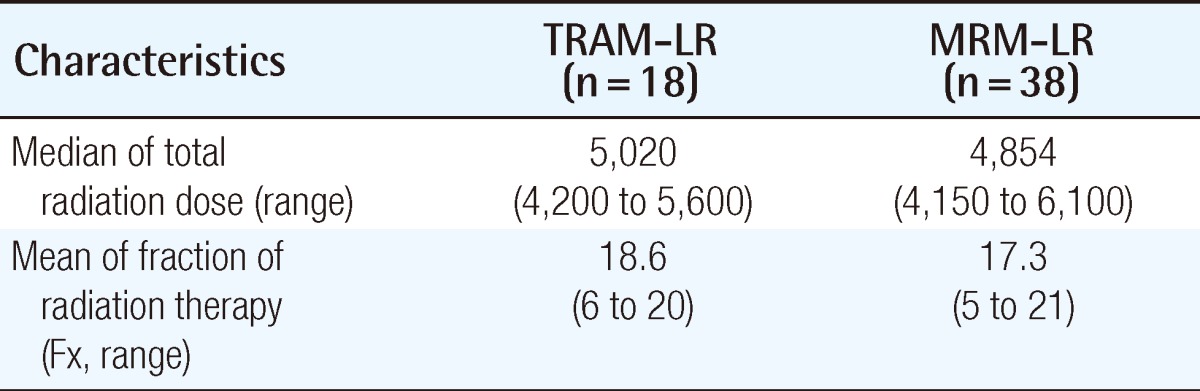
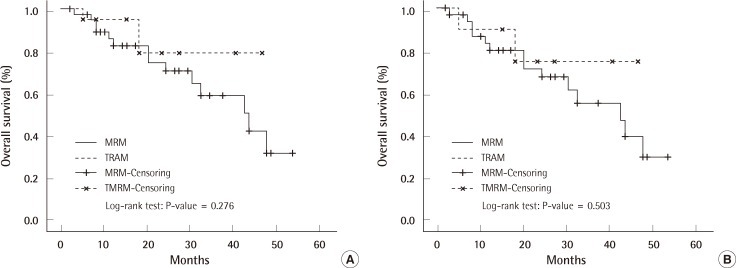
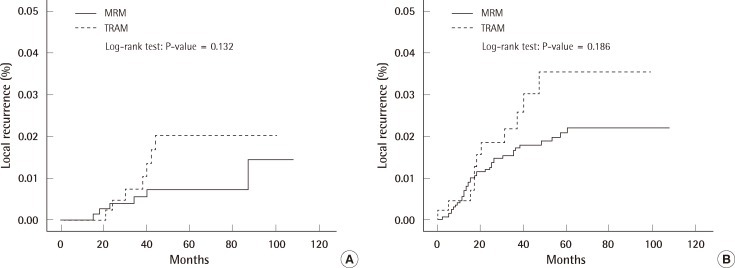
The excised mass, size of wide excision, dose, and period of radiation therapy are shown in Tables 8, 9. The overall survival rate in the TRAM-LR group was higher than that in the MRM-LR group (Table 10) (Fig. 3A). In order to exclude the confounding effect of stage, we analyzed the overall survival rates and categorized them into a lower stage group (stage 0, 1) and higher stage group (stage 2, 3) (Fig. 3B). After excluding the confounding effect of stage, there was no significant difference between the two groups (P=0.503) (Fig. 3B). However, analysis for the lower stage group could not be performed due to the small number of patients. Only one patient in the TRAM-LR group died, which was caused by pericardial effusion after radiation therapy for multiple bone metastases of breast cancer.
Case 1
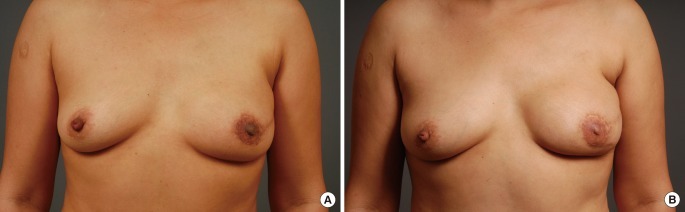
A 43-year-old patient underwent immediate TRAM breast reconstruction for stage 1 invasive ductal carcinoma. Skin-sparing mastectomy was performed for the reconstructive surgery. There was no lymph node metastasis. After surgery, the patient underwent 4 cycles of chemotherapy with doxorubicin and cyclophosphamide. Three years after the TRAM operation, the patient visited the outpatient clinic in the general surgery department with the chief complaint of a palpating mass. Local recurrence developed on the skin in the left lower quadrant of the left breast as a mass sized at 1 cm in the largest diameter in the subcutaneous layer of the reconstructed breast. The excised mass was 2.5×2.3×1.5 cm, weighed 3.3 g, and was diagnosed as invasive ductal carcinoma. After wide excision of the involved tissue, primary closure of the defect could be performed by the oncologic surgeon (general surgeon) without performing a skin graft; the patient then underwent radiation therapy (60.4 Gy/32 Fx). Two years after the wide excision, she is still alive with her breast mound maintained (Fig. 4).
Case 2
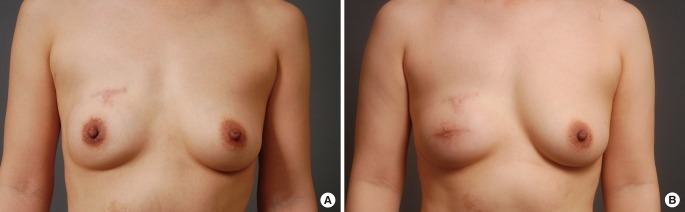
A 39-year-old patient underwent immediate TRAM breast reconstruction for stage 0 invasive ductal carcinoma. A nipple areolar-sparing mastectomy was performed for the first reconstructive surgery. There was no adjuvant therapy. The patient visited the outpatient clinic in the general surgery department with the chief complaint of bleeding from the right nipple. Breast cancer recurred on the skin of the periareolar site in her right breast 2 years after surgery. The largest diameter of the recurrent mass was 1.2 cm. The nipple was excised. The excised mass was 4.2×2.5×2 cm and weighed 8 g. After wide excision of the involved tissue, primary closure of the defect was carried out by the oncologic surgeon (general surgeon) without requiring a skin graft. There was no involvement of the margins or distant metastasis. The patient did not undergo radiation therapy or chemotherapy after wide excision. Two years after the wide excision, she is still alive with her breast mound maintained (Fig. 5).
Case 3
A 28-year-old patient underwent immediate TRAM breast reconstruction for stage 1 invasive ductal carcinoma. A nipple areolar-sparing mastectomy was performed for the first reconstructive surgery. The patient underwent 4 cycles of chemotherapy with doxorubicin (Adriamycin) and cyclophosphamide. A skin lesion around the previous scar site was detected during a regular follow-up at the outpatient clinic in the general surgery department 1 year after the first reconstructive surgery. The largest diameter of the tumor was 1.6 cm. The excised mass was 4.2×1.8×1.3 cm and weighed 10 g. Wide excision was performed, and primary closure was carried out by the oncologic surgeon (general surgeon) without performing a skin graft. There was no involvement of the margins or distant metastasis. The patient did not undergo radiation therapy or chemotherapy. Three months after the secondary operation, the breast mound and projection were maintained (Fig. 6).
DISCUSSION
Local recurrence after breast reconstruction is not always predictable. An oncologic or reconstructive surgeon can encounter such cases. Treatment of local recurrence after immediate breast reconstruction has been reported by Newman et al. [6] who claimed that the rate of local recurrence is low and the likelihood of local control and survival is high. Howard et al. [7] confirmed that local recurrence after TRAM flap reconstruction can be effectively managed with surgical excision of the involved tissue, chemotherapy, and radiation therapy. Our data demonstrated that excellent local control of the disease can be achieved with multimodality therapy including limited surgery without removing the entire breast mound. In our study, the local recurrence rate was not significantly different between the MRM group and TRAM group after stage 4 was excluded (Table 3), which supports previous studies [5,8-12]. This study also confirmed that after excluding the effect of the stage as a major confounding factor, there was no significant difference in the local recurrence rates between the MRM-LR group and TRAM-LR group (Fig. 2A, B).
The TRAM flap did not delay the detection of local recurrence (Table 6). Newman and colleagues [6] reported that among the 23 cases of local recurrences from the M.D. Anderson Cancer Center, 96% presented in the skin or subcutaneous tissue with only one recurrence deep within a TRAM flap, which was detected using a chest radiograph. Similarly in our patients, the most common recurrence site was the skin (72.2%) (Table 5). This shows that the TRAM flap does not interfere with the detection of local recurrence in most cases. Getting a second opinion from an oncologic surgeon or plastic surgeon might have affected these results. The overall survival rate did not significantly differ between the MRM-LR group and TRAM-LR group (Fig. 3). Even after excluding the effect of the stage, the overall survival rate was not significantly different between the MRM-LR group and TRAM-LR group (Fig. 3).
Our study showed that immediate TRAM breast reconstruction did not interfere with the detection of local recurrence and did not affect the overall survival rates after treatment of local recurrence. We assume that our plastic and oncologic surgeons' preoperative counseling in term of selecting patients and follow-up is adequate and also that local recurrence after immediate TRAM breast reconstruction can be at least as effectively managed.
Aesthetically, the breast mound was well maintained after wide excision in most of the cases in the TRAM-LR group. For surgical modalities, most patients in the TRAM-LR group could undergo surgery by simple excision and primary closure. Out of the 18 patients who required a skin graft, only one had Paget's disease, but it was still possible to maintain her breast shape. On the other hand, 4 patients in the MRM-LR group needed skin grafts. Apart from its invasiveness and donor site morbidity, a skin graft on a bare chest wall leaves a surface that is more vulnerable to irradiation, which is often necessary in case of local recurrence. Breast reconstruction with autologous tissue such as a TRAM flap builds a mound with sufficient skin and soft tissue, which allows for primary closure following wide excision in most cases without severely changing the shape of the breast. Interestingly, at the time of wide excision, the mass sizes were smaller and weighed less in the TRAM-LR group than the MRM-LR group, which might be due to the regular follow-up visits and early detection of local recurrence.
When the patients in TRAM-LR group were asked, after undergoing wide excision, whether they would recommend the reconstructive procedure to their friends or not, 12 (85%) out of 14 replied they would recommend this procedure to others. This result shows that the patients in the TRAM-LR group were generally satisfied with the outcome even after local recurrence after immediate TRAM breast reconstruction.
In conclusion, immediate TRAM breast reconstruction following skin-sparing mastectomy does not affect the local recurrence rate in breast cancer, and local recurrence after immediate TRAM flap breast reconstruction could be detected without delay and managed effectively using multiple modalities without reducing overall survival rates. Breast mound reconstruction with soft autologous tissue allowed for primary closure in most cases and all of the patients who survived maintained the contour of their reconstructed breast.
Notes
This article was presented at the 68th Congress of The Korean Society of Plastic and Reconstructive Surgeons on Novermber 4-7, 2010 in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.