Effects of NecroX-5 on the Survival of a Random Pattern Skin Flap in Mice
Article information
Random skin flaps have been widely used for loss of skin and subcutaneous tissues for thousands of years [1]. Unlike the axial pattern flap, the blood supply of a random pattern flap is based on small unnamed vessels. Most unnamed vessels are part of the subdermal plexus [2]. Partial or complete skin flap necrosis is a common problem encountered postoperatively. Flap necrosis caused by disruption of blood supply within the flap often occurs after the surgery, and requires a secondary operation [12].
Researchers have focused on enhancing flap viability to avoid potential necrosis of the flaps [123]. Various pharmacological and surgical delay procedures may be used for enhancement of skin flap viability [3].
NecroX, a member of a novel class of small molecule necrosis/necroptosis inhibitors, inhibits receptor-interacting protein (RIP) kinase-induced necroptosis and is a mitochondrial reactive oxygen species (ROS) scavenger [45]. It has exhibited cytoprotective activity against various insults [45].
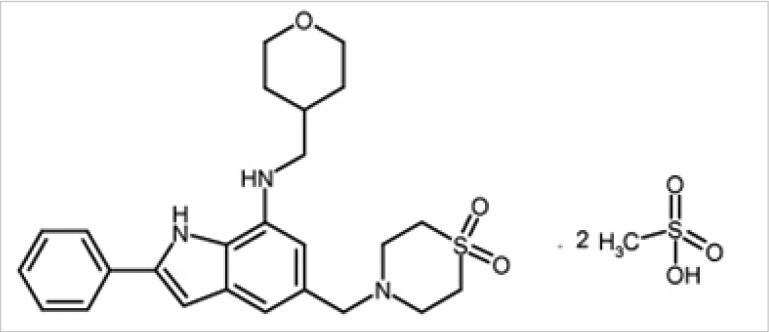
NecroX-5 is a derivative of the NecroX series compounds, with chemical composition C25H31N3O3S.2CH4O3S and molecular weight 453.61 (Fig. 1) [4]. Therapeutic effects of NecroX-5 in ischemia and reperfusion injury of the canine liver and murine heart models have been evaluated [4]. Despite its anti-necrotic effects, no studies on the effect of NecroX compounds on the survival of the skin flap have been reported. The purpose of this experimental study is performing topographical evaluation of the effect of NecroX-5 on the viability of random pattern dorsal skin flaps in mice.
All animal experiments were conducted according to the guidelines for animal experimentation of the author's institution and with their approval. Thirty BALB/c mice (female, 8-week-old, 16.63±0.32 g body weight) were used. They were distributed randomly into three equal groups with 10 mice each. Group I received daily intraperitoneal administration of 10 mg/kg NecroX-5 (LG Life Sciences) in distilled water from 1 day before surgery until 7 days postoperatively, while group II received NecroX-5 at a higher concentration (30 mg/kg in distilled water). Mice in the control group were administered the same amount of distilled water instead of NecroX-5, with a volume of approximately 0.2±0.013 mL.
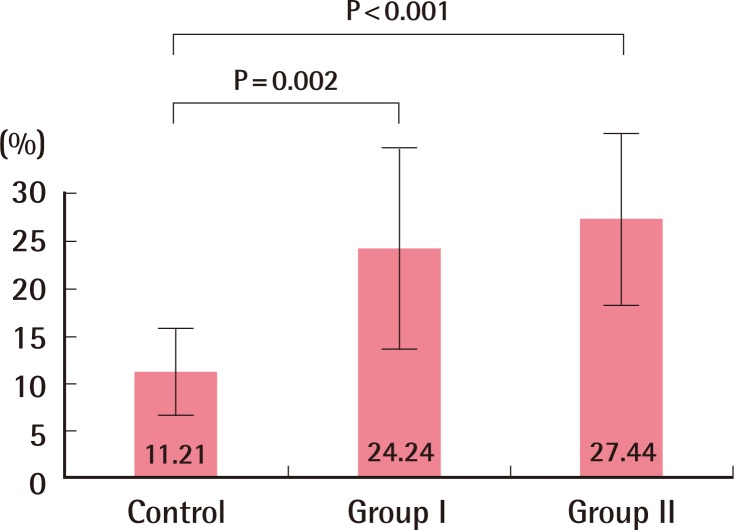
A cranially based random pattern skin flap measuring 1.0 cm×3.0 cm was elevated under the panniculus carnosus layer on the dorsum of each mouse. They were anesthetized intramuscularly with a combination of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (12.5 mg/kg). In order to achieve severe ischemic skin flap conditions, the flap was designed not to include any major pedicles arising from the deep circumflex iliac vessels or lateral thoracic vessels. After flap elevation, the base of the fascia was covered with a silicone sheet, and it was sutured back in place with 6-0 nylon. Surgical procedures were performed under sterile conditions. The mice were kept individually in separate cages in a room with controlled light and temperature and received a standard environment. Seven days after surgery, the mice were anaesthetized and the area of the surviving portion of the skin flap was measured. The animals were sacrificed by high-dose ketamine after digital photographs had been taken. The surviving area was determined grossly by the presence of scab formation, alopecia, and loss of elasticity. The percentage of the area that survived was calculated on postoperative day 7 by digital imaging (Nikon D7000, Nikon Corporation, Tokyo, Japan). Digital photographs of the skin were taken, and the images were transferred to a computer (Fig. 2). The area was calculated as a percentage of the total flap area using Image J Software (National Institutes of Health image). IBM SPSS ver. 21.0 (IBM Co., Armonk, NY, UAS) was used for statistical analysis. The data were presented as mean±standard deviation. Differences among the 3 groups were assessed by Kruskal-Wallis and Mann-Whitney tests. If P<0.016 (adjusted by Bonferroni post-test), it was regarded as statistically significant. The mean percentages of surviving area in the flap, which were measured on postoperative day 7 using the Image J Software program, varied from 5.26% to 22.55% (mean±standard deviation [SD], 11.21%±5.16%) in the control group; 9.71% to 40.03% (mean±SD, 24.24%±10.23%) in group I and 12.39% to 39.19% (mean±SD, 27.44%±8.73%) in group II. The Kruskal-Wallis test showed there was a difference between any pair of groups out of the three (P=0.001). Therefore, the Mann-Whitney U test was applied to determine the differences. The P-value between the controls and group I was 0.002, and between the controls and group II was <0.001 (Fig. 3). However, there was no difference between group I and group II (P=0.529). Additionally, the authors performed the Jonckheere-Terpstra test to observe the dose-response relationship. The result was significant (P=0.001, if P<0.005, it was significant.)
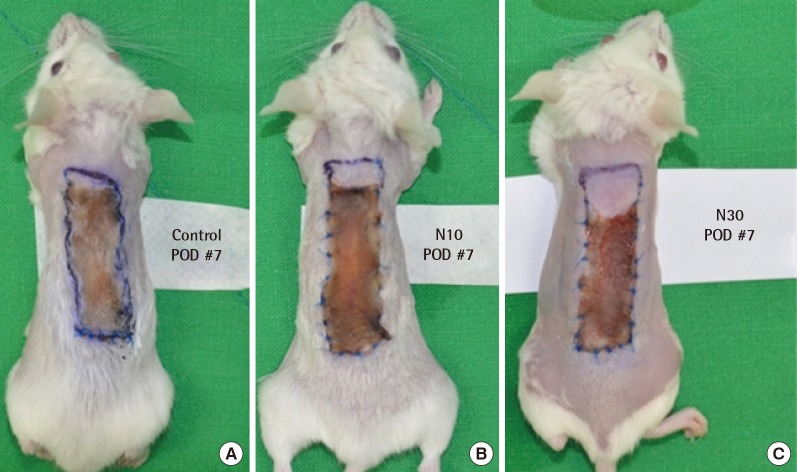
Photographs of random pattern skin flaps. Control group (A), NecroX-5 treatment group I (B) and group II (C) on postoperative day 7. The necrotic areas were significantly larger in the control group than the NecroX-5 treated groups. POD, postoperative day.
The experiment showed a significant flap survival benefit in the NecroX-treated group. Group I and Group II had no significant differences, but the authors believe that if the sample size was larger, the results could have been different. A dose-response relationship was ascertained. This means the chemical has a possibility of more extensive flap survival with higher doses. To the best of the authors' knowledge, most chemical experiments in plastic surgery are focused on increasing angiogenesis or dilation of vessels in a flap model. Few tests were performed to identify the effect of decreased injuries of the flap. This experiment suggests a new trend in experimentation within plastic surgery. Decreasing the extent of insult would be beneficial.
NecroX can decrease various cellular insults. NecroX already demonstrated decreased cell necrosis in a canine hepatic ischemic-reperfusion model [4]. It also decreases cell necrosis against toxic materials [5]. The mechanism of increased flap survival might come from the above. We were not able to identify a more specific mechanism and detailed test, but this brand-new chemical has a very wide range of applications such as free flaps, fat grafts, and even solid organ transplantations. The authors are planning a new in vitro experiment model to identify the exact mechanism against ischemia.
Notes
This work was supported by the 2015 Yeungnam University Research Grant.
This article was presented at the 4th Basic Reconstruction Conference of the Korean Society of Plastic and Reconstructive Surgery on April 4, 2014, in Busan, Korea.
No potential conflict of interest relevant to this article was reported.