Considerations for the Management of Medial Orbital Wall Blowout Fracture
Article information
Abstract
Recently, diagnoses of and operations for medial orbital blowout fracture have increased because of the development of imaging technology. In this article, the authors review the literature, and overview the accumulated knowledge about the orbital anatomy, fracture mechanisms, surgical approaches, reconstruction materials, and surgical methods. In terms of surgical approaches, transcaruncular, transcutaneous, and transnasal endoscopic approaches are discussed. Reconstruction methods including onlay covering, inlay implantation, and repositioning methods are also discussed. Consideration and understanding of these should lead to more optimal outcomes.
INTRODUCTION
Nowadays, medial orbital blowout fracture (BOF) operations have become more common. Rapid developments in medical imaging technology over recent years have resulted in computed tomography (CT) becoming a standard tool for the diagnosis of facial trauma [1], and in the more frequent detection and operation of medial BOF [23].
Patients with medial BOFs usually have nonspecific symptoms, such as periorbital edema, ecchymosis, and subcutaneous emphysema. Generally, the immediate symptoms of medial BOFs are not as serious as those of an inferior fracture because injuries of the extraocular muscles or nerves or soft tissue incarcerations are not as common [4]. Functional and aesthetic sequelae, however, such as extraocular muscle disorders, diplopia, and/or enophthalmos could also occur as they do with inferior BOFs. When physicians encounter a patient with periocular trauma, they should suspect the possibility of medial BOF and undertake a radiologic evaluation. The surgeon should preferentially check the axial and coronal cuts of CT scans, because the plain radiographs, such as Caldwell's and Waters' views, show just nonspecific images of medial wall fractures like haziness of the ethmoidal sinus.
The purpose of this article is to review medial BOF treatment with emphasis on anatomy, fracture mechanisms, surgical approaches, and reconstruction materials and methods.
ANATOMY AND MECHANISM
The medial orbital wall is composed of the frontal process of the maxilla, the lacrimal bone, the orbital plate of the ethmoid, and the lesser wing of the sphenoid, through which the optic nerve traverses in the optic canal. The lamina papyracea, supported by honeycomb-like bony septa of the ethmoid sinuses, constitutes the largest, main portion of the medial wall of the orbit [5], and has a convex orientation with respect to the orbital cavity. Pneumatized ethmoid air cells, which maintain structural stability and resist fractures of the medial orbital wall, act as a safeguard for the eyeball during trauma. The foramina of the anterior and posterior ethmoidal arteries are located along the fronto-ethmoidal suture line [6], and the anterior ethmoidal artery, posterior ethmoidal artery, and optic canal have been reported to be at approximately 24 mm, 36 mm, and 42 mm from the medial orbital rim [78]. Surgeons should be well acquainted with this anatomy and keep in mind not to dissect over the posterior ethmoid foramen to avoid optic neuropathy [9]. The optic neuropathy can be caused by direct nerve injury or retinal arteriolar occlusion. Careless dissection near the optic ring could tear soft tissues around the optic nerve and cause them to swell. This swelling in the limited bony canal compresses retinal vessels and may induce optic nerve ischemia and resultant optic nerve injury. The optic nerve can also be injured by compression of unrecognized hematoma around the retrobulbar space. For this reason, many experienced surgeons recommend not dissecting over the posterior ethmoidal foramen [1011].
The mechanism of medial BOF could be largely explained by hydraulic pressure; that is, increased pressure in orbital soft tissue acts on the orbit to outfracture the medial orbital wall [1213]. The buckling mechanism is a relatively uncommon cause of medial BOF [14]. This bone conduction theory holds that direct trauma to the rigid orbital rim transmits force posteriorly to cause compression fracture of the orbital wall [1516]. On the other hand, there is another old theory called the "direct globe-to-wall contact" mechanism suggested by Raymond Pfeiffer in 1943. This hypothesis means that when the globe is displaced posteriorly by forces, the fracture is possible with exactly the same displacement of the globe like a footprint [1718].
Surgeons should consider the kinetics of injury and choose a treatment plan after meticulous clinical examination and precisely analyzing CT images. When a fracture is extensive and enophthalmos is anticipated, surgery is usually recommended within 2 weeks of trauma to prevent soft tissue scarring and contracture in a nonanatomic position [19]. The indications for early surgery are diplopia caused by soft tissue incarceration, a positive forced duction test, a significant change in globe position, and a compressed optic canal [2021].
SURGICAL APPROACHES
Transcaruncular approach
The transcaruncular approach is favored by many surgeons for the reconstruction of medial BOF, because it enables medial orbital wall defect exposure without leaving cutaneous scars [222324].
Equipment, such as a headlight and loupes, enable bright and clear visualization of the operative field to simplify surgery. The use of a corneal protector is mandatory during this approach to prevent corneal injury, and a transparent corneal protector permits the early detection of mydriasis and the prevention of optic neuropathy around the optic ring [25]. Through an incision between the caruncle and plica semilunaris conjunctivae, soft tissue dissection is performed up to the periosteum of the medial orbital wall, just behind the rim. Pulling soft tissue upward and downward with small, long retractors, the freer is used for subperiosteal dissection until the area of the fracture is well detected, while avoiding any damage to the lacrimal sac and medial rectus muscle.
This approach provides direct and predictable access to the medial orbit and avoids injury to the canthal tendon and lacrimal apparatus. However, it has the disadvantage of a limited surgical field, which can make placing of a large implant difficult.
Transcutaneous approach
The percutaneous, subbrow, or upper eyelid crease approaches could be used via a small linear, curvilinear, Z, or vertical incision [262728]. The orbicularis oculi muscle is split carefully, preserving the supratrochlear nerve, the hole is enlarged by retractors and periosteal elevator, and a periosteal incision is made along the edge of the medial orbital rim. This method is reliable and predictable for experienced plastic surgeons, but has the disadvantages of leaving a visible scar and possible injury of the medial canthal tendon or the lacrimal apparatus, and numbness of the medial forehead caused by supratrochlear nerve injury [2930].
Subciliary incision is a familiar method of accessing the lower half or lower two thirds of the medial wall, but it is not easy to obtain full exposure of the operative field, due to soft tissue tethering to the lacrimal sac and inferior oblique muscle. When multiple upper and middle facial fractures are combined, the surgeon could choose a bicoronal incision, which provides excellent visualization for reduction and enables calvarial bone harvesting.
Transnasal approach
The transnasal endoscopic approach is another useful tool for repairing fractured bone segments [31]. With visualization of the inner site of the middle meatus, the mucosa of the uncinate process is incised and the process removed to obtain wide access to the fractured wall. Opening the anterior ethmoidal cells then exposes any fractured bones of the lamina papyracea and herniated orbital contents. The transnasal endoscopic approach provides good aesthetic results without an external scar [3233]. Its disadvantages include difficulties associated with implant insertion and reconstructing large defects. In addition, there is a learning curve and possibilities of recurrence of BOFs when there are comminuted fractures [34]. Recently, Lim et al. [10] reported good surgical outcomes using a combination of transorbital and transnasal approaches that mobilize the fractured orbital wall back to its original position through transnasal manipulation without endoscopic equipment.
RECONSTRUCTION MATERIALS
It is well known that the type of material used for orbital reconstruction is less important than the methods in which materials are used. However, the surgeon should decide what materials to use when confronted with an extensive large defect, combined associated injuries, or a pediatric patient, as well as when donor morbidity is considerable [35].
Ideal orbital implants are biocompatible, available in sufficient amounts, strong enough to provide orbital support, easily shaped, and radiopaque. Various repair materials have been introduced for the treatment of medial BOF [35], and available graft materials can be categorized as autologous and alloplastic.
Autologous materials include bone, cartilage, fascia lata, and periosteum. Autologous bone grafts are favored because of their availability, mechanical properties, revascularization potential, and low risks of infection or scarring [363738]. Donor sites include calvarium, iliac crest, rib, anterior wall of the maxillary sinus, mandibular symphysis [39], and autologous cartilage grafts, such as conchal, septal, or rib cartilage are also candidates. However, autologous materials have unpredictable resorption rates and provide suboptimal volume correction and their malleabilities result in grafts that are less than architecturally accurate. In addition, their utilities are limited by associated donor site morbidity [4041].
To date, several alloplastic materials, such as titanium, porous polyethylene, resorbable materials, gel film, bioglass, and silastic sheeting have been introduced [424344]. These materials eliminate donor site morbidity and reduce operative time, and they are readily available [4546]. Porous polyethylene is an inert, nonabsorbable polymer formulated to contain a network of open and interconnecting pores of 100–250 µm in size; it facilitates tissue ingrowth and reduces foreign body reactions and capsule-associated complications. In fact, vascular and soft tissue ingrowth through its pores can be observed at 1 week after implantation without fixation.
Titanium has been used extensively in the craniofacial surgery and dentistry fields in the form of implants, plates, and screws. Titanium is biocompatible, has excellent physico-mechanical properties, and is strong, rigidly fixable, widely available, relatively familiar to surgeons, and is osseointegrated with minimal foreign body reaction [47], and thus, it could be an ideal implant for covering large anatomical defects. However, titanium is costly and if not cut properly may have irregular edges that impinge on soft tissues. Furthermore, wider incisions are necessary when covering large defects with titanium plates. Hybrid products, such as the titanium reinforced polyethylene fan implant (SynPOR titanium orbital mesh plate, Synthes Inc., West Chester, PA, USA) can also be used.
Bioactive silica glass is bacteriostatic and more rigid than other materials and has potential as an orbital implant, but it is difficult to customize [48].
Silicone sheeting has been widely used for orbital reconstruction. The positive attributes of this material include low cost, flexibility, and ease of handling, while providing adequate support for maintaining orbital contents for even large orbital fractures. Its disadvantages are its lack of rigidity, the possibilities of fibrous capsule formation, infection, and extrusion. Due to the proximity of the medial orbital wall and the mucosa of the ethmoidal sinuses, formation of a capsule could significant increase the risk of infection.
Poly-L-lactide (PLLA) and polyglycolic acid (PGA) are absorbable implants with sufficient biomechanical resistance for orbital wall reconstruction. Both are malleable and impermanent and in time are replaced by bone. The drawbacks of PLLA and PGA implants are radiolucency, the possibility of inflammation with degradation, limited durability, and low strength, and thus, the adoption of PLLA implants for the treatment of large orbital wall defects has been limited.
RECONSTRUCTION METHODS
Various methods have been devised to reconstruct the medial BOF, and surgeons may have opportunities to gain valuable new skills to debunk surgical myths.
The onlay covering method
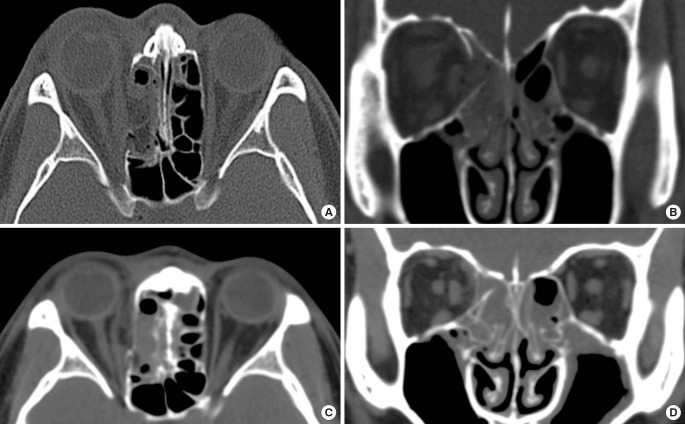
The onlay covering method is a widely used, accepted, and effective means of treating for medial BOF [49]. It includes the following procedures: circumferential subperiosteal dissection, reduction of herniated orbital contents, and wider dissection than the defect area followed by onlay covering with thin materials to complete orbital continuity (Fig. 1). When a defect is large, a longer incision and wider dissection are required to provide sufficient space for a large implant to cover the entire defect. However, when orbital bone support is inadequate, inserted materials can sink or be displaced, which increases the risk of BOF recurrence.
Inlay implantation
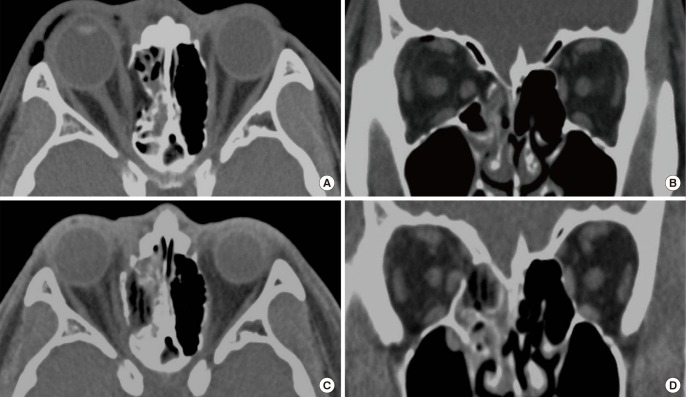
Inlay implanting methods involve insertion of implant materials layer-by-layer into the ethmoidal sinus to achieve medial orbital wall continuity. After transcaruncular incision and restoring herniated soft tissue, a 3 mm thick porous polyethylene plate is cut into pieces and inserted into the sinus through the defect area (Fig. 2). The advantage of this method is simple and safe. The main surgical procedure is performed in the ethmoidal sinus and not the orbital cavity. This provides adequate restoration of premorbid orbital volume even for comminuted fractures involving the posterior medial wall to the posterior ethmoid foramen, avoiding the possibility of optic nerve injury. As the ethmoidal sinus is filled with implants, one could question whether the blockage of the drainage and infection of the ethmoidal sinus might occur. The inlay implantation technique is not completely filling the ethmoidal sinus but partially rebuilding the orbital continuity, leaving enough space for drainage. The porous polyethylene has excellent biocompatibility providing fibrous tissues and blood vessels to grow into the pores, resisting any infection [23].
Inlay implantation
The fractured bone segments were removed and several pieces of 3-mm thick porous polyethylene plate were inserted into the defect area of the ethmoidal sinus. (A, B) Preoperative computed tomographic axial view and coronal view. (C, D) Postoperative computed tomographic axial view and coronal view.
The repositioning method
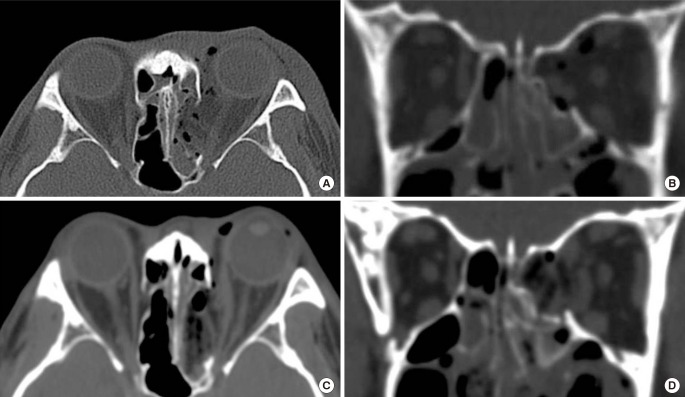
The fractured bone segments of the medial orbital wall can be preserved with periosteum and repositioned in their original positions (Fig. 3). The endoscopic transnasal approach provides an effective means of repositioning fractured segments [3132] and of maintaining the medial orbital wall in the preinjury position until bone union. When a fracture segment is large and green-stick displaced, the bone segment could be repositioned transcaruncularly. The slender freer is inserted through the fracture line into the ethmoidal sinus, and then by pushing out the tip of the freer from under the displaced segment, the fractured medial orbital wall can be repositioned by leverage. Furthermore, inserting implants into the ethmoidal sinus under the bone segment prevents BOF recurrence. However, it is not recommended for comminuted fracture because small fractured segments could be displaced later.
UP-TO-DATE TECHNIQUES
The determination of the shape of an implant required to treat an extensive orbital fracture is sometimes not straightforward. Recently, preoperative computer-assisted planning using virtual correction models and patient-specific implants using CT mirror images of the non-affected orbit have been introduced [50]. This procedure could reduce operative times and provide more accurate reconstruction on an individual anatomic basis.
Intraoperative navigation surgery using three-dimensional (3D) CT is also useful for complex orbital fractures [51]. This modality involves the synchronous positioning of instruments with 3D CT images [52], and enables accurate, safe surgery on orbital fractures around the optic canal based on visualizing locations indicated by the intraoperative navigation pointer. Intraoperative CT [53] and the intraoperative 3D C-arm [54] helpfully identify current implant positions and confirm immediate postoperative orbit status.
Orbital volumes can be measured using CT information and software packages, such as Aquarius Workstation (iNtuition Aquarius, version 4.4.6; TeraRecon Inc., San Mateo, CA, USA) and automated rapid stereolithography machine (SLA3500, 3D Systems, Rock Hill, SC, USA) [55]. These volume measurement methods provide objective assessments for preoperative planning and of postoperative outcomes.
POSTOPERATIVE CARE
Bedside ophthalmologic examinations, which include visual acuity and diplopia, extraocular muscle movement assessments are required after medial orbital wall reconstruction. Intra- and postoperative CTs are useful for assessing surgical results, by providing exact locations of implants, restored soft tissue (including extraocular muscle), and repositioned bone fragments. The patient should be placed in the Semi-Fowler position and informed to avoid physical stimulations, such as, bumps, hard exercise, eye rubbing, and nose blowing, until the medial orbital wall has stabilized. Regular postoperative follow-up is recommended at intervals of several months to assess the degree of enophthalmos, which could be checked with a Hertel or Naugle exophthalmometer or volumetric analysis of CT data at least 1 year postoperatively.
CONCLUSIONS
Although medial BOF is less symptomatic than inferior BOF, it may lead to serious sequalaes. The goal of medial BOF reconstruction is restoration and maintenance of premorbid orbital cavity volume. Many surgeons have been devoted to finding a better way to achieve optimal outcome in fields of the medial BOF. The optimal surgical outcomes could be achieved when the surgeon gave sufficient consideration to orbital anatomy, the fracture mechanism, surgical approaches, reconstruction materials and surgical methods.
Notes
No potential conflict of interest relevant to this article was reported.