Bilateral Atypical Ductal Hyperplasia with Microcalcifications in a Patient with Gynecomastia
Article information
The incidence of male breast cancer is far lower than that of breast cancer in female patients, and usually has a worse prognosis. The incidental discovery of breast cancer in patients undergoing surgery for gynecomastia is rare, but has previously been reported by several authors [1]. However, bilateral atypical ductal hyperplasia in men is far less frequent, and this is the first report to describe the occurrence of this finding with associated microcalcifications in the histopathological analysis.
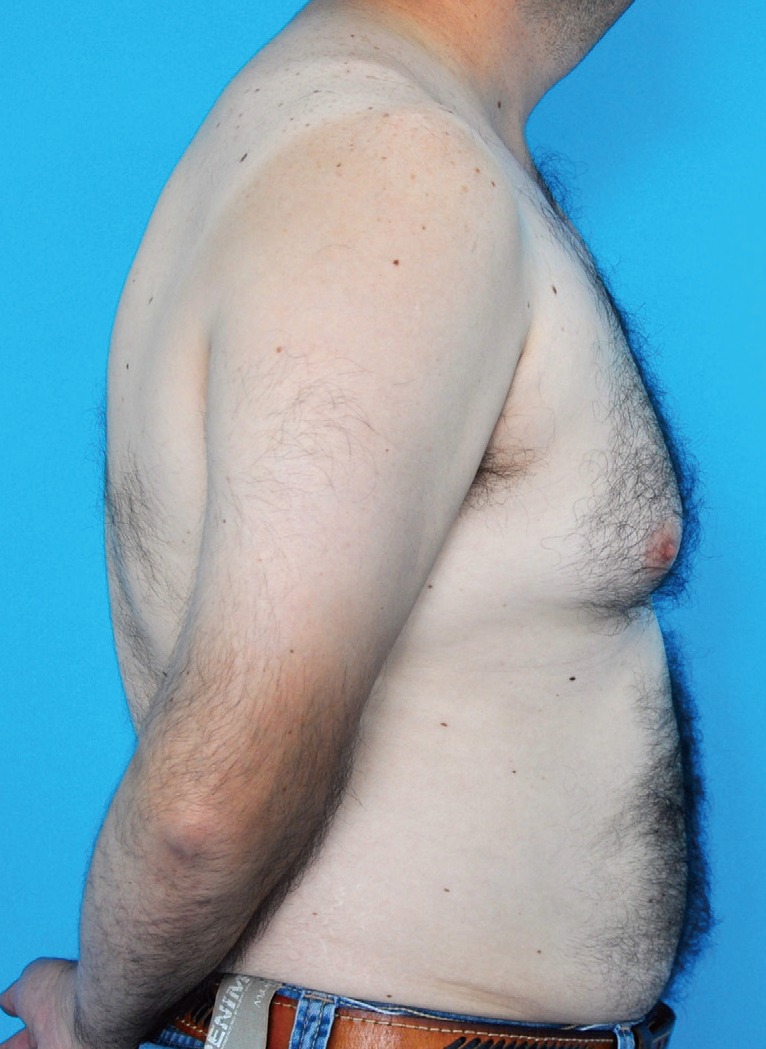
We present the case of a 39-year-old male patient who attended a plastic surgery consultation complaining of long standing bilateral gynecomastia (Fig. 1). He had no personal history of any significant medical conditions or family history of breast cancer. He also denied the use of medication and recreational drugs. Physical examination revealed bilateral breast enlargement with palpable glandular tissue below and surrounding the nipple-areolar complex, with adjacent fat tissue. A testicular examination was normal.
Routine preoperative laboratory tests did not show any alterations in transaminase, plasma creatinine, or thyroid hormone levels. The presence of bilateral glandular breast tissue was confirmed using ultrasound imaging.
A combined technique was used to correct this patient's gynecomastia by performing tumescent liposuction of the thorax followed by transareolar resection of the breast tissue. The operation was uneventful, with 45 g of glandular tissue on the left side and 23 g on the right side. The surgical outcome and the patient's postoperative course were satisfactory, and he was discharged the following day without further complications (Fig. 2).
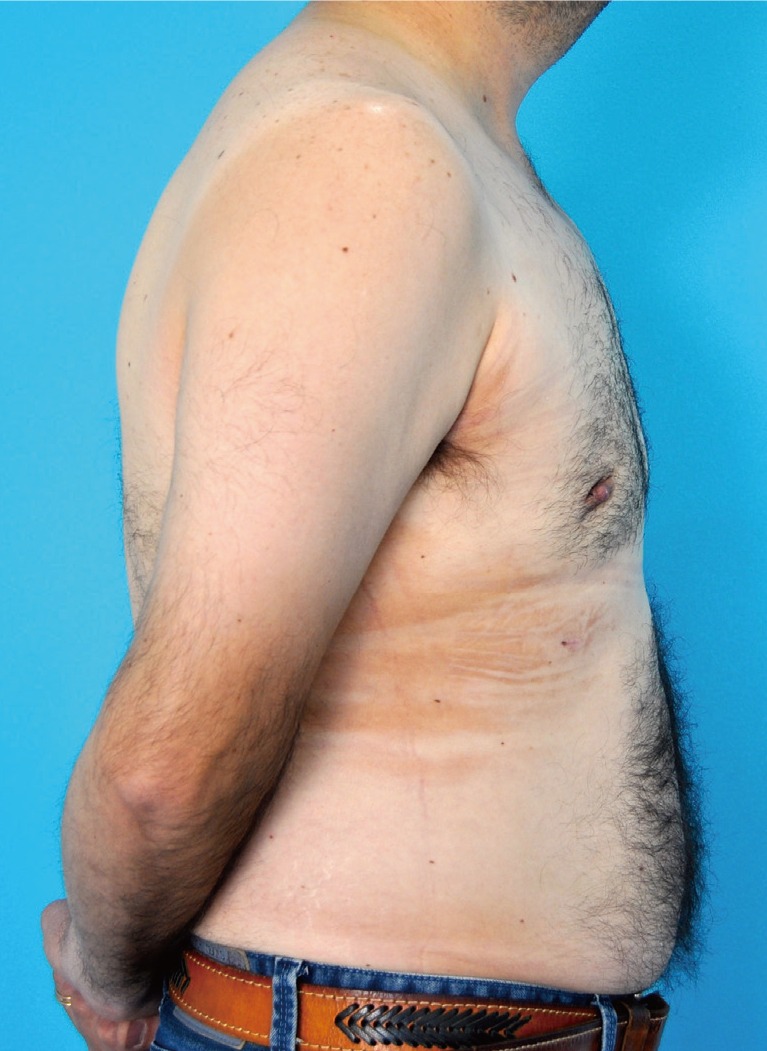
Postoperative photographs six weeks after surgery. Although the swelling had not completely resolved at this stage, the satisfactory nature of the results can be appreciated.
The histopathological analysis reported several samples in which stromal fibrosis with hyaline parts was found. Irregular ductal structures were identified, inlaid with hyperplastic epithelium following a cribriform pattern. The cells contained hyperchromatic, pleomorphic, and enlarged nuclei with minimal overlaying and well-defined cytoplasmic borders. Some calcium carbonate microcalcifications were also identified in both samples. No evidence of intraductal or invasive neoplasia was found (Fig. 3).
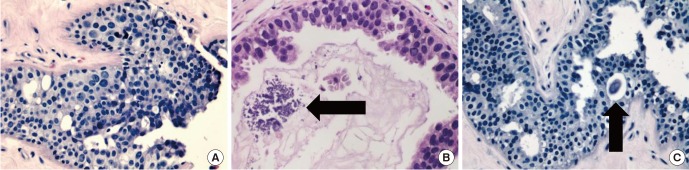
(A) Hematoxylin and eosin (H&E)-stained section in which ductal structures can be identified following a cribriform pattern, with cells showing varied degrees of atypia (×100). (B, C) Microcalcifications (black arrows) can be seen as groups of granular basophilic material inside the lumen of the previously described mammary ductal structures (B, ×400; C, ×100, respectively).
Male breast cancer accounts for 1% of all cases of breast cancer. Although no causal relationship between gynecomastia and male breast cancer has been shown, it is a common association. Up to 40% of patients with male breast cancer present with gynecomastia, most likely because elevated estrogen levels are present in both conditions [1]. Due to its lower prevalence, screening for male breast cancer in the general population it is not advised. However, imaging tests are an important part of the preoperative evaluation of patients with gynecomastia, not just for screening purposes but also to differentiate between true gynecomastia and pseudogynecomastia. Although mammography is widely available and has a specificity and sensibility of greater than 90% for finding malignancies in the male breast, it is usually uncomfortable and poorly tolerated by men with mild breast enlargement. Ultrasound is a reliable alternative that does not involve those disadvantages of mammograms, and has the advantage of allowing ultrasound-guided biopsies if needed [2].
Atypical ductal hyperplasia is a disorder that increases the risk of suffering from both ipsilateral and contralateral breast cancer by up to five times in female patients. It is usually found incidentally in biopsies of suspicious mammographic abnormalities. The current recommendations are to provide advice about cancer risk reduction strategies to female patients suffering from this condition. Breast examinations every six months and yearly mammography are indicated, along with the avoidance of oral contraceptives and hormone replacement therapy.
Microcalcifications are not a rare phenomenon, as they are associated with a wide range of breast conditions, such as benign, precancerous, and cancerous lesions. The presence of microcalcifications aids in the histopathological diagnosis of female breast biopsies because they are associated with comedonecrosis in intraductal neoplasms [3]. Furthermore, core biopsies with microcalcifications are more likely to be reported as cancer than are samples without microcalcifications.
To our knowledge, this is the first report of atypical ductal hyperplasia with microcalcifications in a male patient [4]. Consequently, insufficient evidence exists to support the hypothesis that this condition is a risk factor for male breast cancer, as it is for females. This explains why no current recommendations exist for the care of these patients. In the case presented in this article, the patient was referred to a breast cancer specialist for annual follow-ups with ultrasound imaging.
Without the routine histopathological analysis of the resected specimens from gynecomastia surgery, it would have been impossible to detect this premalignant condition. This standard of practice is of the foremost importance not just for this reason, but also in order to accurately diagnose male breast cancer as early as possible in a larger number of patients.
Notes
This study was presented at the 12th Chilean Congress of Breast Surgery on September 27th–29th, 2015, in Pucon, Chile.
No potential conflict of interest relevant to this article was reported.