Mandibular Reconstruction Using the Free Vascularized Fibula Graft: An Overview of Different Modifications
Article information
Abstract
The reconstruction of the mandible is a complex procedure because various cosmetic as well as functional challenges must be addressed, including mastication and oral competence. Many surgical techniques have been described to address these challenges, including non-vascularized bone grafts, vascularized bone grafts, and approaches related to tissue engineering. This review summarizes different modifications of the free vascularized fibula graft, which, since its introduction by Hidalgo in 1989, has become the first option for mandibular reconstruction. The fibula free flap can undergo various modifications according to the individual requirements of a particular reconstruction. Osteocutaneous flaps can be harvested for reconstruction of composite defects. 'Double-barreling' of the fibula can, for instance, enable enhanced aesthetic and functional results, as well as immediate one-stage osseointegrated dental implantation. Recently described preoperative virtual surgery planning to facilitate neomandible remodeling could guarantee good results. To conclude, the free fibula bone graft can currently be regarded as the "gold standard" for mandibular reconstruction in case of composite (inside and outside) oral cavity defects as well as a way of enabling the performance of one-stage dental implantation.
INTRODUCTION
The reconstruction of the mandible is a complex procedure and continues to be a challenge in reconstructive craniomaxillofacial plastic surgery. Indications for mandibular reconstruction are versatile, and include oncologic resections, traumatic injuries, and osteoradionecrosis [12]. The mandible serves several important functions in the head and neck. The ultimate goal is restoration of both form and function, necessitating the evaluation of appearance, mastication, deglutition, speech, and oral competence. This can be achieved through a variety of surgical techniques, mainly non-vascularized and vascularized grafts. Non-vascularized bone grafts (NVBGs) may be suitable in specific circumstances, like delayed reconstructions of small traumatic bony defects. They provide a framework for creeping substitution. Yet the lack of blood supply results in slow and incomplete healing, as well as increased rates of infection, non-union, and fracture [34]. They are also prone to osteoradionecrosis in conjunction with radiation therapy [5].
The advent of microvascular surgery has revolutionized mandibular reconstruction, especially after cancer treatment utilizing radiation. Vascularized bone grafts (VBGs) contain an intrinsic blood supply that adds the biological advantage of shortened union time. Outcomes from free VBGs, most notably free fibula grafts, have proved markedly superior to non-vascularized options, including reconstruction plates and bone grafts, with defects of the mandible exceeding 6 cm in length and traversing the parasymphyseal and/or anterior border regions [34].
Direct comparisons of NVBGs and vascularized bone flaps (VBFs) have shown superiority of the latter in terms of bony union (69% for NVBGs vs. 96% of VBFs) [3] as well as superior functional and aesthetic scores for diet, speech, and midline symmetry [6]. Superiority of VBGs compared to NVBGs increases significantly in case of mandibular defects greater than 6 cm or previously irradiated tissue [3]. Available options for VBGs are the fibula, radial forearm, scapula, and iliac crest [78910], with the fibula flap being the most popular for mandibular reconstruction.
In this review, the authors present different variations of the free vascularized fibula graft, which could serve as the treatment of choice for mandibular reconstruction.
FIBULA FLAP
The use of free vascularized fibula has become the "gold standard" for mandibular reconstruction since its introduction by Hidalgo [11] in 1989, due to various advantages over other VBGs. First, the fibula graft offers a good length of dense cortical bone, up to 25 cm in adults, as well as a long pedicle based on the peroneal artery for the reconstruction of long bony defects. Since the fibula graft was first described, many surgeons have adopted and ultimately optimized the technique. Today, multiple modifications exist, each one developed to fit in a specific individualized defect scenario.
Bone-only and osteoseptocutaneous flap
A few circumstances exist in which there is no associated soft tissue damage and an osseous flap only is sufficient to bridge the defect. Composite tissue loss is usually more extensive, and the versatility of the fibula flap comes into play.
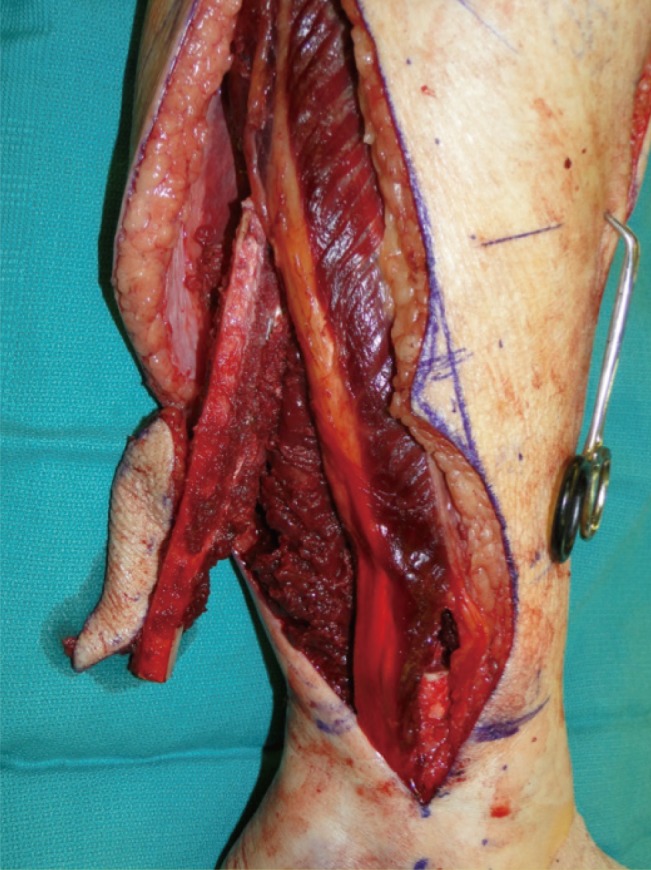
The fibula graft may provide skin islands, up to 25 cm long and 14 cm wide, suitable for reconstruction of associated soft tissue defects (Fig. 1). Chen and Yan [12] were the first to describe the osteocutaneous fibula flap in 1983. Multiple skin islands can be harvested with the fibula graft, including those based on septocutaneous as well as on musculocutaneous peroneal perforators supplying an osteomyocutaneous flap [1314]. The most reliable septocutaneous perforators are located in the middle and distal third of the fibula [15]. Modifications have been developed that include parts of the soleus or peroneus longus. Most recently, a second skin island based on proximal perforators off the peroneal artery present in 90% of the cases was described [16]. Thus, this graft provides convenient tissue for simultaneous reconstruction of bony and soft tissue defects inside as well as outside the oral cavity, bringing viable tissue to a mostly irradiated and contaminated field, with the lowest complication rate among osteocutaneous flaps [17]. Limitations of the extent of the skin paddle harvested with the fibula graft have been overcome with the previously described double-skin paddle fibula free flaps based on septocutaneous, intramuscular, and soleus perforators (Fig. 2) [1819].
In-situ osteoseptocutaneous fibula flap
Osteoseptocutaneous fibula flap immediately after harvest, including the skin paddle. The skin island is located between the middle and distal third of the fibula (markings).
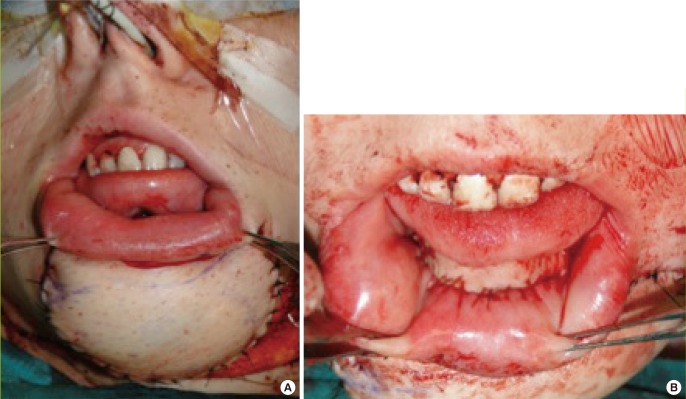
Double-skin island osteoseptoctaneous fibula free flap
In this patient after major mandibular resection for osteosarcoma, the double-skin island osteoseptocutaneous fibula free flap is used for simultaneous reconstruction of a soft tissue defect externally (A) and intraorally (B). The photograph depicts the immediate reconstructive result at the end of the procedure.
Double barreling
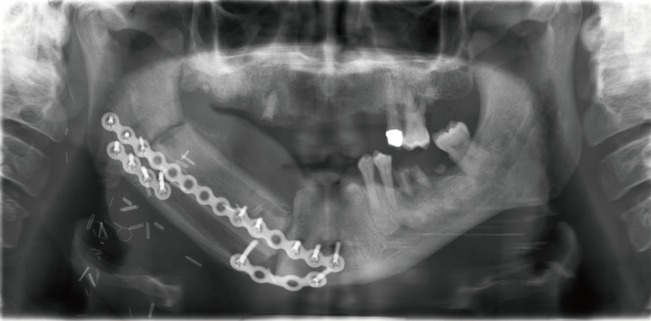
The height discrepancy between the native mandible and the transplanted fibula can be a disadvantage of this approach, especially at the anterior segment, which significantly complicates prosthodontic reconstruction of the mandible. The average height of the native mandible, including the dentition, is generally in excess of 3–4 cm. The average height of the fibula is usually 10–12 mm, which presents a reconstructive dilemma, as this height is inadequate to allow for placement of dental implants that can be restored in a functional setting. 'Double-barreling' of the fibula, a technique that involves osteotomies and folding over the fibula graft to create equal struts, while preserving the blood supply throughout the graft, was first recommended by Horiuchi et al. [20] in 1995. A double-barrel fibula flap conveniently matches the height of the mandible of 3–4 cm, leading to better aesthetic and functional results and enabling a one-stage procedure with immediate osseointegrated dental implantation (Fig. 3) [2122]. In terms of dental implantation, the vertical distraction technique has been described to expand the neomandible up to 15 mm to allow a delayed osseointegrated dental implantation after 4–6 months in a three-stage procedure [2]. Most recently published data have shown a increased complexity and frequency of complications with the vertical distraction technique compared to double-barreled fibula grafts and therefore recommend the use of double-barreled fibula grafts for osseointegrated dental implants [23]. Depending on the reconstructive purpose, the fibula can also be partially double-barreled [2]. A 98% flap survival and good aesthetic and functional outcomes have been reported [24].
Pre-plating and osteotomies
To recreate the anatomic form of the mandible, especially the contour of the anterior portion, the fibula has to be divided into several segments using a closed wedge osteotomy technique with potentially multiple osteotomies. The number of osteotomies should be kept to a minimum to preserve reliable segmental periosteal circulation [25]. The accurate restoration of the neomandible is crucial due to sufficient occlusion and acceptable aesthetic results. The contour of the neomandible will be defined principally by the curve of a reconstruction plate formed prior to the surgery or during the surgery using a no-touch technique along the specimen. Another technique includes pre-plating of the mandible prior to the resection. Different variations of pre-plating are described depending on the tumor spread [26]. The stereolithographic model completes this technique involving preplating, templating, and insetting of the mandible with convincing results with regard to shape and function [27].
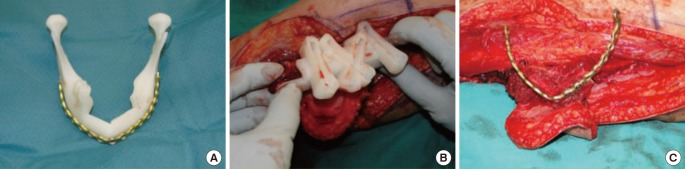
Prefabricated plates and cutting systems—three-dimensional approach
As described before, sculpting the fibula graft is a critical step during restoration of the mandibular shape. The evolution of technology has introduced three-dimensional (3D) approaches to virtual surgical planning by preparation of 3D models of fibula grafts prior to surgery. Stereolithographic models of the mandible and the fibula can be obtained from computer tomography scan data preoperatively [28]. Subsequently, plates can be bent according to the model to increase the accuracy and decrease the duration of surgery by preplanning each step of the operation including the osteotomies on the mandible and the fibula by use of staged cutting guides (Fig. 4) [29]. Thus, computer-assisted mandibular reconstruction (CAMR) has increased preoperative planning accuracy, resulting in greater surgical precision and reduction of surgical duration [30]. However, these models are expensive, require a strict realization of the planned intervention, and are not easily adaptable to an aberrant surgical approach [31].
Future directions
Utilization of microsurgery in management of the mandibular reconstruction, even though associated with adequate outcomes and low donor-site morbidity [32], it remains challenging. Future strategies include tissue engineering approaches utilizing collagen-based scaffolds combined with bone marrow-derived stromal cells and growth factors [533]. Furthermore, particulate cellular bone and marrow (PCBM) and platelet-rich plasma (PRP) has been shown to form microstructures of the cellular bone resembling those in normal mandibles [34]. Additionally, an off-label use of bone morphogenetic protein-2 (rhBMP-2) in a collagen carrier has been described as a new alternative to various types of autogenous bone grafting procedures [3536]. Nevertheless, none of these approaches can yet be considered established as standard in craniomaxillofacial reconstructive surgery.
DISCUSSION
The reconstruction of mandibular defects is complex due to the need to address both functional and aesthetic objectives. This review has noted that reconstruction with a free fibula bone graft has been shown to produce superior patient outcomes over NVBGs [34]. Nevertheless, it is also one of the most challenging procedures from the standpoint of craniomaxillofacial surgery. For this reason, NVBGs may be a reasonable alternative in selected cases, depending on the size of the bony and soft tissue defect [3738]. Additionally, soft tissue flaps alone such as anterolateral thigh, gracilis, rectus, and latissimus dorsi have been successfully used in reconstruction of posterolateral defects [3940]. Tissue-engineered alternatives have also shown promise but still need more comprehensive clinical testing. In the case of additional soft tissue defects, a fibula graft can be harvested with various reliable skin paddles [1], which enable a one-stage reconstructive procedure of composite mandibular defects [414243]. Several modifications of skin paddles have been described, including a chimeric double-skin paddle [18] for simultaneous reconstruction of intra- and extraoral defects [44]. Especially in such complex defects, the free fibula graft has outstanding importance. Another advantage of the free vascularized fibula graft is the ability to have two teams working simultaneously with the patient in the supine position by reducing operating time, which is associated with reduced blood loss and lower rates of infection [45]. Additionally, the blood supply can be monitored postoperatively with an implantable Cook-Swartz Doppler probe, since the peroneal artery remains sizeable, enabling the recognition of vascular compromise early, which in turn results in an overall flap success rate of 98.1% [4647]. The donor site morbidity of the free vascularized fibula graft is consistently acceptable among different studies, and is mostly avoidable with careful planning and appropriate technique [484950].
One disadvantage of the free fibula graft is the height discrepancy between the native mandible and the transplanted fibula, especially at the anterior segment. The 'double-barreling' of the fibula to create equal struts is a useful modification with good aesthetic and functional outcomes [24]. Partially double-barreled grafts, as needed for aesthetic improvements, have also been reported, and a commonly used modification [2]. The 'double-barreling' of the fibula enables immediate osseointegrated dental implantation, obtaining better results and lower complication rates compared to vertical distraction devices [23]. Levine et al. [51] even published three cases of total mandibular reconstruction in a single stage procedure with immediate dental implantation. Preoperative virtual surgery planning using 3D technology has shown convincing improvements in postoperative outcomes, including a reduced operation duration and increased functional as well as aesthetic results, and should be considered, especially in complex defects [52]. However, as discussed before, these models are expensive and have not become an integral part of the surgical approach [31].
Notes
No potential conflict of interest relevant to this article was reported.