Use of the Fix and Flap Approach to Complex Open Elbow Injury: The Role of the Free Anterolateral Thigh Flap
Article information
Abstract
Background
Complex elbow injuries with associated nerve, muscle, or joint injury commonly develop post-inury stiffness. In order to preserve function, joint congruency, elbow stability and durable wound coverage must be achieved in a timely manner.
Methods
A retrospective review of patients who underwent orthopaedic fixation followed by free anterolateral thigh (ALT) flap soft tissue coverage was performed. Five patients were identified and included in this study.
Results
We present a series of 5 cases managed with this principle. Soft tissue defects ranged in size from 4×9 cm (36 cm2) to 15×30 cm (450 cm2) and were located either posteriorly (n=4) or anteriorly (n=1). Associated injuries included open fractures (n=3) and motor nerve transection (n=2). Wound coverage was achieved in a mean duration of 18.8 days (range, 11 to 42 day). There were no flap failures and no major complications. The mean postoperative active elbow motion was 102° (range, 45° to 140°).
Conclusions
In our small series we have highlighted the safety and utility of using the free ALT flap in complex elbow injuries. The ALT flap has many advantages which include abundant skin and subcutaneous tissue; vascularised vastus lateralis muscle that was used in our series to obliterate dead space, provide a vascular bed for nerve grafts and combat infection; and, access to fascia lata grafts for reconstruction of the triceps tendon.
INTRODUCTION
The elbow joint is prone to develop post-injury stiffness and its prevention is of paramount importance for a successful outcome. The final outcome in patients with complex elbow injuries is largely dependent on the extent of the initial injury [1]. Soft tissue defects less than 40 cm2 are associated with the greatest return of motion [2]. Larger defects and more complex injuries with associated nerve, muscle, or joint injury are associated with higher degrees of stiffness. In order to preserve function, joint congruency, elbow stability and durable wound coverage with a flap are needed. Joint mobility must be maintained by early motion. The flap should allow some gliding between the skin and underlying joint and should not hamper secondary surgical procedures such as capsulectomy and osteotomy. The purpose of this article is to present our approach to complex elbow injuries and share our experience using the free anterolateral thigh (ALT) flap for soft tissue coverage.
METHODS
Surgical technique
Thorough debridement of the wound and initial stabilisation with external fixation, if needed, is performed. Static external fixators are used temporarily to stabilize the joint until the patient is ready for definitive fixation. Meanwhile, negative pressure wound therapy is used to seal the wound and prevent infection.
Definitive fixation aims to restore articular congruity, re-establish anatomic mechanical axes, and obtain enough fracture stability to allow early, unrestricted elbow range of motion. The patient is deemed ready when the wound is healthy and free of infection. This decision is made jointly with the orthopaedic surgeons.
The ALT flap is harvested as previously described [3]. A fasciocutaneous perforator flap or musculocutaneous flap is harvested based on the reconstructive needs. A musculocutaneous flap is preferred when we need to cover bulky hardware, obliterate dead space or when bone or nerve grafting is performed. The skin paddle is designed so that it is of sufficient size to cover the anastomosis and close the defect without tension. The proximal brachial artery is the preferred site of anastomosis and end-to-side using a slit arteriotomy is performed to preserve the distal run-off [4]. Venous anastomosis is done end-to-end to the venae comitantes of the brachial artery or the basilic vein. Alternatively, the radial or ulnar arteries can be turned up for the arterial anastomosis, and they are usually used in conjunction with the basilic or cephalic vein for venous drainage.
Flap harvest is done concurrently with fixation. During inset of the flap, care is taken to ensure complete muscle wrap of hardware, bone and nerve grafts. Any dead space is obliterated with vascularised muscle. Postoperatively the elbow is splinted in 30° of flexion and elevated. Mobilisation exercises are started after 7 days.
Patients
Case 1
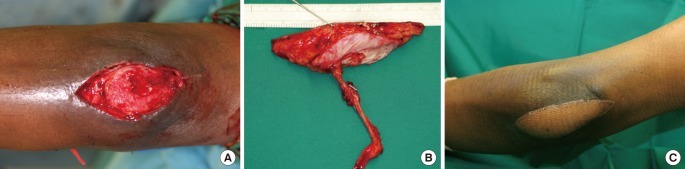
A 40-year-old male was a driver of a car that overturned in a road traffic accident. He sustained a Gustilo IIIB open fracture dislocation of the right olecranon with degloving injury and 10 cm segmental loss of the right ulnar nerve (Fig. 1A, B). Initial management included multiple wound debridement and external fixation. 11 days after injury, internal fixation of the olecranon was performed. The ulnar nerve gap was bridged with a 3-cable sural nerve graft (Fig. 1C, D). A free ALT musculocutaneous flap was used to cover the elbow. The nerve grafts were buried in the forearm flexor/pronator muscle mass and covered with vastus lateralis muscle. The affected limb was immobilized in extension for 7 days. Despite using a flap with very well vascularised muscle, due to the severity of the injury and degree of comminution, he later developed septic arthritis and osteomyelitis necessitating removal of the implants. Heterotopic ossification developed subsequently. At latest review at 9 years 2-point discrimination was 7 mm, he had regained full hand function (intrinsic muscle power 4/5) and active range of motion at the elbow was 45° (range, 45° to 90°) (Fig. 1E, F). He declined further surgery.
Gustilo IIIB fracture of the olecranon, degloving injury and 10 cm ulnar nerve loss
(A) Open comminuted fracture dislocation of the elbow with temporary external fixation. (B) Radiograph of the right elbow after internal fixation with 3.5 mm reconstruction plate. (C) Segmental ulnar nerve loss (yellow arrows indicate nerve ends). (D) Ulnar nerve was repaired with a 3-cable sural nerve graft buried within flexor/pronator muscle complex. (E, F) Nine year follow-up. Elbow range of motion: 45° (range, 45° to 90°). Ulnar nerve motor recovery grade M4. Patient demonstrates good hand opening and abduction of digits (E) as well as strong power grip (F).
Case 2
A 34-year-old car driver was involved in a road traffic accident. He sustained a Gustilo IIIB open fracture dislocation of the right capitulum and radial head, segmental loss of the radial nerve, and anterior elbow skin loss measuring 15×10 cm. Surgical debridement was repeated until the wound was clean. Reduction of the radial head, sural nerve grafting for the radial nerve gap and free ALT flap coverage was performed 15 days after the initial trauma. No recovery of radial nerve function was observed and tendon transfers to restore wrist extension were performed. Eight months post elbow reconstruction his active elbow motion was 110° (range, 0° to 110°).
Case 3
A 40-year-old male was a front seat passenger in a car involved in a road traffic accident. He sustained fractures of the left 1st to 6th ribs, a left sided pneumothorax, pneumomediastinum, left scapula fracture, dislocation of the left little finger PIPJ as well as a crush injury of the left elbow with open fracture of the left humerus and proximal ulna. A chest tube was inserted and the little finger reduced. The elbow wound underwent debridement followed by external fixation and negative pressure dressing. Internal fixation of the humerus and ulna and soft tissue coverage with a free ALT flap were performed 13 days following injury. Nineteen months postoperatively active elbow motion was 115° (range, 15° to 130°) (Fig. 2).
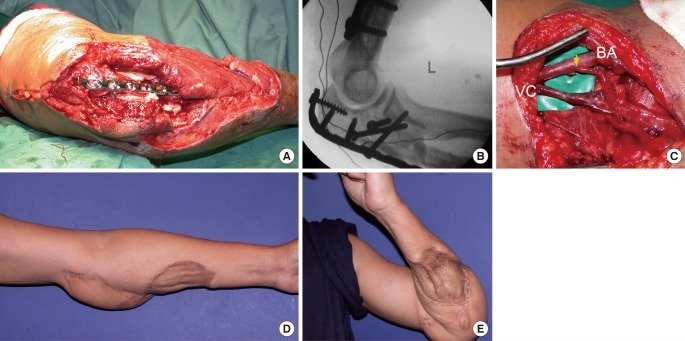
Crush injury of the left elbow with open humerus and ulna fractures
(A) Open transverse humeral fracture, comminuted olecranon fracture with internal fixation with contoured low contact dynamic compression (LCDC) plates. (B) Internal fixation with contoured LCDC plates. (C) End-to-side arterial anastomosis (yellow arrow) to the brachial artery (BA) to preserve distal circulation. Venous anastomosis to the vena comitans (VC). (D, E) Nineteen month follow-up. Elbow range of motion: 115° (15° to 130°).
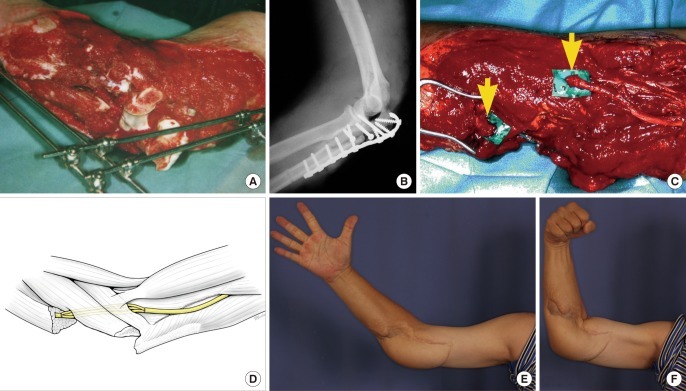
Case 4
A 22-year-old prison inmate sustained a closed fracture of his right olecranon after a fall. Operative fixation of his fracture was complicated by wound infection, breakdown and bony non-union. Mycobacterium tuberculosis was isolated from the wound. After multiple wound debridements and negative pressure wound therapy, the triceps tendon was anchored to the olecranon and reinforced with a tensor fascia lata graft, immediate soft tissue coverage was achieved with a free ALT flap. A sinus developed at the flap edge that resolved after debridement and antimycobacterial treatment. At the most recent follow-up 2 years post reconstruction he can actively flex his elbow 100° (range, 30° to 130°) (Fig. 3).
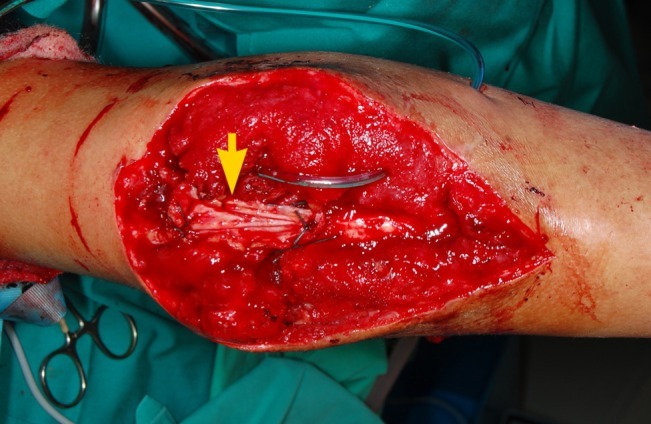
Triceps tendon reconstruction and ALT flap coverage
Infection of previous fixation of olecranon fracture resulting in septic arthritis and wound breakdown. Picture shows secondary reconstruction of the triceps tendon with a rolled fascia lata graft (yellow arrow). The soft tissue defect was successfully covered with a free anterolateral thigh (ALT) musculocutaneous flap (not shown).
Case 5
A 61-year-old man sustained a left olecranon avulsion fracture after he tripped and fell, landing on his left elbow. His repair was complicated by staphylococcus aureus wound infection requiring removal of hardware, debridement and negative pressure wound therapy. A free ALT perforator flap based on the oblique branch of the lateral circumflex femoral artery was used for soft tissue coverage once the infection was cleared. Two months after coverage active elbow flexion is 140° (range, 0° to 140°) (Fig. 4).
RESULTS
A summary of patients, treatments and results can be found in Table 1. The mean age of the patients was 39.4 years (range, 22 to 61 years). All were male. The defects were secondary to trauma (n=3), post-surgical infection (n=2) was the other cause. Soft tissue defects ranged in size from 4×9 cm (36 cm2) to 15×30 cm (450 cm2) and were located either posteriorly (n=4) or anteriorly (n=1). Associated injuries included open fractures (n=3) and motor nerve transection (n=2). All cases had vital structures to be covered-orthopaedic hardware (n=2), exposed bone (n=4), or exposed triceps tendon (n=1). Fasciocutaneous (n=1) and musculocutaneous flaps (n=4) were used. Arterial revascularisation was performed end-to-side to the brachial artery (n=4) and end-to-end to a recurrent branch of the brachial artery (n=1). Wound coverage was achieved in a mean duration of 18.8 days (range, 11 to 42 days). Minor complications included wound infection (n=2) and heterotopic ossification (n=1). There were no flap failures and no major complications. Postoperative wound infection One patient suffered a prolonged local infection with mycobacterium tuberculosis. The mean postoperative active elbow motion was 102° (range, 45° to 140°).
DISCUSSION
In this series of patients with medium to large elbow defects, the goals of stable internal fixation and durable skin coverage were achieved by collaboration between the orthopaedic and plastic surgeons. Successful coverage allowed early aggressive joint mobilisation.
Orthopaedic fixation was used to treat bony and soft tissue injury, and soft tissue coverage was performed when the wound was deemed clean and free of infection. Elbow fractures were reduced and internally fixated with preservation of articular congruity using devices such as the 3.5 mm reconstruction plate, 3.5 mm low contact dynamic compression plates as well as anatomical locking plates e.g., proximal ulnar locking compression plates. Successful operative treatment depends on restoring joint congruency (especially the ulnohumeral joint) and elbow stability, so that early joint motion is possible [5]. Dead space at the fracture site can be a problem if the implants do not conform exactly to the bone surface or if there is a bone gap. A well vascularised muscle can fill the dead space and reduce the risk of infection.
Many local and pedicled flaps have been described for elbow reconstruction. Local flaps are indicated for small defects with healthy adjacent skin. Common designs include rotation flaps, transposition flaps, and perforator based island flaps. However, they cannot be used if there is extensive degloving injury. Pedicled flaps such as the radial foream, ulnar forearm, antecubital fasciocutaneous, and posterior interosseous flaps have been used, however, the disadvantages are extensive scarring and the need for skin grafting over the forearm. Muscles such as flexor carpi ulnaris, brachioradialis, and anconeus can be elevated to provide coverage of small areas but at the sacrifice of some limb function [6]. The pedicled latissimus dorsi flap is a traditional workhorse flap for coverage of large elbow defects, especially proximal to the olecranon. When used for defects beyond the olecranon it has a propensity for distal tip necrosis, wound breakdown or failure [2]. Free flaps are indicated for large defects distal to the olecranon [7].
Although free tissue transfer has become increasingly popular, it has not been emphasised sufficiently for elbow coverage. The English literature contains relatively few reports on its use. Hallock used five local fascial flaps and three free flaps for elbow coverage in his series of upper extremity trauma [6]. He advocated using local flaps for mild injuries but maintained that free flaps were necessary in larger or composite defects. Choudry et al. [2] reported using free tissue transfers in only 19% of cases in their series of 96 patients requiring soft tissue coverage of the elbow. Of the 19 free flaps, only five (26%) were ALT flaps, with the latissimus dorsi being the flap of choice (42%). This may stem from the perception that the vascular supply of the ALT flap is unreliable, although recent studies have defined its vascular anatomy and proven the flap's reliability [8,9]. Other than the ALT flap, perforator flaps including the thoracodorsal artery perforator [10-12] and superficial circumflex iliac artery perforator [13] flaps offer reliable skin cover.
The ALT flap is ideal for elbow coverage. It can provide large amounts of skin (up to 35 cm long and 25 cm wide can be harvested on a single dominant perforator [14]) as well as vastus lateralis muscle, which can be used to obliterate dead space and combat infection. Furthermore, vascularised muscle helps to vascularise the sural nerve grafts [15,16] and optimise outcome as seen in case 1. The motor nerve to the vastus lateralis could potentially be used as a vascularised nerve graft [17]. Even if the motor nerve is harvested, or the vastus lateralis muscle is included in the flap, donor site morbidity is acceptable and studies have shown that all patients eventually return to their preoperative level of function [18,19].
Fascia lata grafts are easily harvested at the time of flap elevation and can be used to reconstruct the triceps tendon (case 4) or elbow ligaments. Primary closure of the donor site is possible if the skin paddle is ≤7 to 9 cm wide [14], but the donor site is skin grafted if a larger skin paddle is needed. The skin paddle should cover the vascular anastomoses and be sufficiently large to accommodate the hardware and allow the elbow to move freely. Since the donor site is located a distance from the elbow, harvest of the flap can proceed concurrently with the other elbow procedures. A thin flap can be obtained by elevating in a suprafascial plane [20] or by trimming of the subcutaneous fat to the subdermal level [21,22]. The skin paddle of the ALT flap allows fast skin-to-skin healing at the interface between the flap and the wound edge and its subcutaneous tissue allows gliding between the joint and skin.
Loss of movement after injury to the elbow is common. Sojbjerg [1] defined a stiff elbow as one with flexion of less than 120° and a loss of extension of greater than 30°, Morrey et al. [23] found that most tasks of daily living can be performed with 100° of elbow motion (from 30° to 130°). All of our patients displayed ≥100° of elbow flexion except for patient 1, who had elbow flexion of 45° (range, 45° to 90°). Although his elbow was stiff, he had excellent ulnar nerve recovery and declined further surgery.
The causes of posttraumatic elbow stiffness are varied and can be classified as intrinsic or extrinsic. Heterotopic ossification, as occurred in patient 1, is the most common cause of extrinsic elbow contracture and it is characterised by progressive ossification of periarticular soft tissues [24]. Operative release of the stiff elbow depends on the aetiology but usually involves release of the posterior band of the medial collateral ligament, debridement of any bony blocks to motion and/or anterior and posterior capsulectomy [25]. Total elbow arthroplasty may be considered in selected cases [25]. The ALT skin paddle affords skin-to-skin healing and facilitates secondary surgery of the elbow.
In conclusion, the advantages of the ALT flap for elbow coverage are 1) abundant skin and subcutaneous tissue; 2) vascularised vastus lateralis muscle that can be used to obliterate dead space, provide a vascular bed for nerve grafts and combat infection; and, 3) access to fascia lata grafts for triceps tendon repair.
Notes
This article was present at Chang Gung Mayo Clinic Symposium in Reconstructive Surgery, October 27-30, 2011.
No potential conflict of interest relevant to this article was reported.