A Simple, Reliable, and Inexpensive Intraoperative External Expansion System for Enhanced Autologous Structural Fat Grafting
Article information
Abstract
External volume expansion of the recipient site by suction has been proposed as a way of improving fat graft survival. The objective of this study was to present an innovative and simple intraoperative external expansion system to enhance small-volume autologous fat grafting (40–80 mL) and to discuss its background and its mechanism of action. In this system, expansion is performed using a complete vacuum delivery system known as the Kiwi VAC-6000M with a PalmPump (Clinical Innovations). The recipient site is rapidly expanded intraoperatively 10 times for 30 seconds each with a negative pressure of up to 550 mm Hg before autologous fat injection. During this repetitive stimulation, the tissues become grossly expanded, developing macroscopic swelling that regresses slowly over the course of hours following the cessation of the stimulus. The system sets various mechanisms in motion, including scar release, mechanical stimulation, edema, ischemia, and inflammation, which provide an environment conducive for cell proliferation and angiogenesis. In order to maintain the graft construct in its expansive state, all patients are encouraged postoperatively to use the Kiwi three times daily for one minute per session over the course of three days. The handling of this system is simple for both the patients and the surgeon. Satisfactory clinical outcomes have been achieved without significant complications.
INTRODUCTION
Autologous fat grafting is becoming increasingly popular for soft tissue augmentation and reconstruction, due to improvements in donor site preparation and fat tissue harvesting, processing, and transplanting techniques. Moreover, adipose tissue demonstrates a regenerative potential related to the presence of mesenchymal stem cells [1]. However, the unpredictability of volume enlargement and fat tissue retention continue to pose major problems. Recent studies have investigated the role of the recipient site and its preconditioning in order to address these concerns [23].
Release of the contracture at the time of fat grafting is considered necessary in cases of contracted scar tissue, as seen in breasts with previous infection, radiation scarring, scar contracture from previous surgery, or congenital constriction bands, as seen in tuberous breasts [4]. "Rigottomies," named after Gino Rigotti, who described the technique, are commonly used to release and stretch-expand scars by creating multiple tiny nicks inside the contracted tissue [4]. This technique transforms the scar into a three-dimensional mesh, expanding the volume of the recipient bed, and thereby reducing the interstitial fluid pressure into the adequate range for graft survival [4]. Attention is paid in order to avoid the creation of cavities [4].
Furthermore, the efficacy of pre-expansion external vacuum devices has been investigated in clinical and pre-clinical studies, especially to allow mega-volume (250-mL range) fat grafting in and around the breast [5]. The Brava system, a bra-like external expansion device, has been successfully used by Del Vecchio and Bucky [6] to precondition the recipient site and obtain a 60%–200% increase of human breast volume after autologous fat injection, as documented by quantitative magnetic resonance imaging. Moreover, Heit et al. [2] and Lancerotto et al. [3] demonstrated that external volume expansion applied to mouse integument induces considerable proliferation and vascularization in the subcutaneous tissue, which can be elicited even by a single two-hour external volume expansion cycle.
IDEA
Based on the above-discussed research, we have developed a new method to precondition localized scarred recipient sites intraoperatively in a simple, time-saving, and inexpensive manner. In order to obtain a combination of advantages similar to those described for Rigottomies and for preoperative external expansion systems, we apply an intraoperative external expansion device to prepare the recipient site for small-volume autologous fat grafts (40–80 mL) when the recipient site is characterized by a restrictive cicatrix or pre-irradiated tissues.
Expansion is performed using a complete vacuum delivery system known as a Kiwi VAC-6000M with a PalmPump (Clinical Innovations, South Murray, UT, USA). This vacuum delivery device is an integrated unit, providing an ergonomic handle with a simple hand-vacuum pump, thumb- or finger-activated vacuum release valve, and accurate vacuum indicator (Fig. 1). The device is relatively cheap (62 EUR) and readily available. It is used by gynecologists to extract fetuses. The recipient site is rapidly expanded intraoperatively 10 times for 30 seconds each with a negative pressure of up to 550 mm Hg before autologous fat injection (Fig. 2A, B). During this repetitive stimulation, macroscopic swelling of the soft tissue is observed (Fig. 2C). The recipient scaffold for fat grafts is then loosened and stretched out. This repetitive cycle leads to intense edema, ischemia, and inflammation, and provides an ideal environment for cell proliferation and angiogenesis.
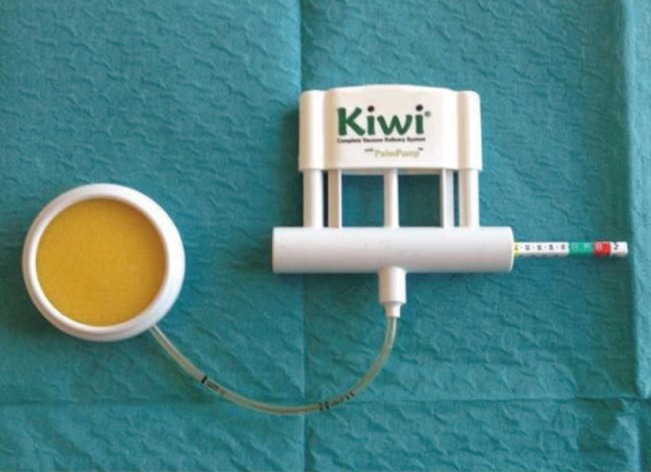
The external expansion device
The complete vacuum delivery system used: Kiwi VAC-6000M with a PalmPump (Clinical Innovations).
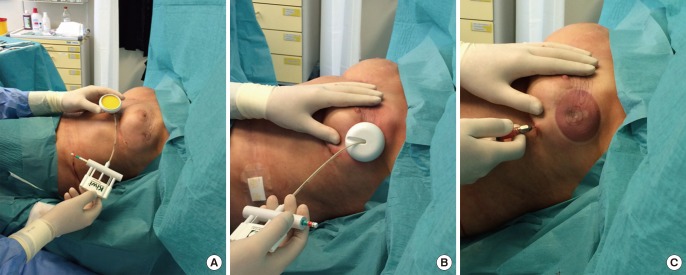
Intraoperative use of Kiwi on scar tissues
(A, B) The Kiwi is applied on a scarred pre-irradiated breast. (C) During the repetitive stimulation, macroscopic swelling of the soft tissue is observed.
In order to maintain the graft construct in its expansive state and further optimize the ideal graft-to-recipient volume ratio, all patients are encouraged to use the Kiwi postoperatively three times daily for a period of one minute per session over the course of three days.
The procedure can be performed under local or general anesthesia and, in our experience, has minimal morbidity and high patient acceptance and compliance. Patients who undergo local anesthesia for the procedure tolerate the device well and report only some moderate discomfort and pressure during application. The handling of the device is simple for both the patients and the surgeon. Postoperatively, some patients experience a small degree of edema, which normally resolves without sequelae.
DISCUSSION
Extreme variability is found in the literature regarding the ideal negative pressure to be applied on the recipient site prior to fat grafting. Several studies have investigated the effects of a negative pressure of -25 mm Hg applied for a single round of two hours or for two hours daily for five days in murine models, resulting in cell proliferation, neoangiogenesis, and neoadipogenesis [127].
A lower pressure (-125 mm Hg) was applied by Lee et al. [8] in an experimental model involving white rabbits. This negative pressure was continuously applied for one week prior to fat grafting. Lee et al. [8] reported improved recipient site vascularization and, accordingly, enhanced fat graft survival.
Finally, in their most recent clinical study of the Brava system, Khouri et al. [9] applied a high-vacuum cycling pump for 10 hours per day with a negative pressure of -60 mm Hg for two to four weeks prior to fat grafting.
In comparison with these reports from the recent literature, we apply a stronger negative pressure (-550 mm Hg) for a much shorter intraoperative period (five minutes). Following the recommendations of Chin et al. [10] and similarly to Khouri et al. [9], we apply cyclical forces to guarantee a more robust response, with the pump cycling between -550 mm Hg for 30 seconds to no pressure.
The rationale of using Kiwi prior to fat grafting is to obtain the release of contracted scar tissues by exploiting its traction force, and to promote intense edema, ischemia, and inflammation in order to provide an ideal environment for cell proliferation and angiogenesis.
We hypothesize that, due to micromechanical strain, the application of pressure to the extracellular matrix results in an increase of cell anchorage to focal adhesion sites of the extracellular matrix fibers, although we are not able to characterize these events at the molecular level. These effects have largely been shown in vitro, where a proliferative state is induced by acting on a gate-control signal on global cellular activity mediated by the cytoskeleton [23].
The short-term use and application of high intraoperative pressure in a limited area may lead to results comparable to those obtained through the long-term use of the device, clinically confirming the experimental observations of Lancerotto et al. [3]. Our technique may be especially useful in cases of restrictive subdermal cicatrix, through the creation of a vascularized scaffold that is seeded with fat grafts. Conversely, it has been shown that the long-term use of pre-expansion has limitations, especially in severely deformed breasts after conservative surgery and radiation therapy, where it may induce skin complications such as dermatitis, pigmentation, or blistering.
In conclusion, based on our experience, we propose that this device is useful for preparing the recipient site for fat grafting, especially in cases where the surgeon must deal with retractions or scarring as a result of radiation therapy (Fig. 3).
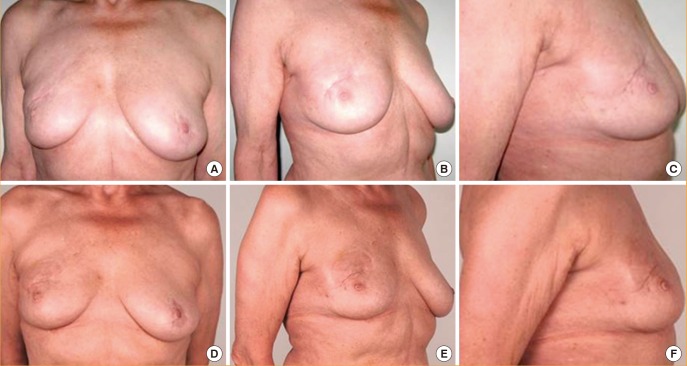
Preoperative and postoperative views
(A-C) Preoperative views of a 67-year-old woman with a complaint of contracted scar tissue on the right breast, two years after breast-conserving surgery and radiation therapy. (D-F) Postoperative views seven days after one fat grafting procedure, with 40 mL grafted after recipient site preconditioning with Kiwi. Release of the scar with volume restoration is visible, although slight edema may be noted in the early follow-up images.
Notes
This article was presented at the International Federation for Adipose Therapeutics and Science in Amsterdam, Holland, November 13th through 16th, 2014; and at the Seventh International Conference on Regenerative Surgery in Rome, Italy, December 10th through 12th, 2015.
No potential conflict of interest relevant to this article was reported.