Clinical Factors Associated with the Non-Operative Airway Management of Patients with Robin Sequence
Article information
Abstract
Background
The indications for surgical airway management in patients with Robin sequence (RS) and severe airway obstruction have not been well defined. While certain patients with RS clearly require surgical airway intervention and other patients just as clearly can be managed with conservative measures alone, a significant proportion of patients with RS present with a more confusing and ambiguous clinical course. The purpose of this study was to describe the clinical features and objective findings of patients with RS whose airways were successfully managed without surgical intervention.
Methods
The authors retrospectively reviewed the medical charts of infants with RS evaluated for potential surgical airway management between 1994 and 2014. Patients who were successfully managed without surgical intervention were included. Patient demographics, nutritional and respiratory status, laboratory values, and polysomnography (PSG) findings were recorded.
Results
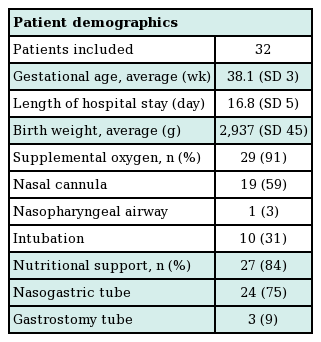
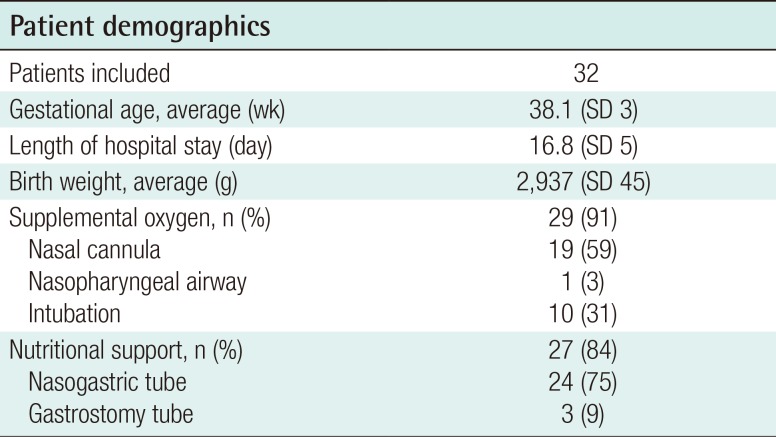
Thirty-two infants met the inclusion criteria. The average hospital stay was 16.8 days (range, 5–70 days). Oxygen desaturation (<70% by pulse oximetry) occurred in the majority of patients and was managed with temporary oxygen supplementation by nasal cannula (59%) or endotracheal intubation (31%). Seventy-five percent of patients required a temporary nasogastric tube for nutritional support, and a gastrostomy tube placed was placed in 9%. All patients continued to gain weight following the implementation of these conservative measures. PSG data (n=26) demonstrated mild to moderate obstruction, a mean apneahypopnea index (AHI) of 19.2±5.3 events/hour, and an oxygen saturation level <90% during only 4% of the total sleep time.
Conclusions
Nonsurgical airway management was successful in patients who demonstrated consistent weight gain and mild to moderate obstruction on PSG, with a mean AHI of <20 events/hour.
INTRODUCTION
Robin sequence (RS), which involves the clinical triad of micrognathia, glossoptosis, and upper airway obstruction, affects approximately 1 in 8,500 births [1]. Infants may present with widely varying phenotypes, ranging from infrequent episodes of respiratory obstruction and/or feeding difficulty to severe crises of asphyxia and failure to thrive [2]. Treatment options vary to a similar extent, from conservative modalities—namely, prone positioning and nasopharyngeal airway (NPA) placement [3456]—to operative interventions, including the subperiosteal release of the floor of the mouth [7], tongue-lip adhesion [891011121314], tracheostomy [1516], and mandibular distraction osteogenesis [1718192021222324]. Predicting which patients will improve with conservative measures versus which patients require an operative intervention is challenging in the clinical context.
The indications for surgical intervention are vague and subjective, and are often based upon nonspecific clinical findings. Prior studies have developed grading systems, clinical criteria, and algorithms to aid in therapy selection, but these systems remain incomplete in identifying those patients who will likely improve without surgery [25]. Overall, little consensus exists regarding objective, empirically proven clinical parameters for choosing between operative and non-operative airway management.
The purpose of this study was to review the clinical course and characteristics of patients with RS at a large tertiary care center whose airway was successfully managed without surgical interventions. This information may help avoid unnecessary surgical airway procedures in patients with RS when the decision to operate or not to operate may be unclear.
METHODS
A retrospective review was undertaken of the records of patients with RS who underwent primary airway management at our institution from 1994 to 2014. After obtaining approval from the Institutional Review Board, the study cohort was identified by performing a search using International Classification of Diseases, version 9 (ICD-9) codes and current procedure terminology (CPT) codes in a long-standing prospective craniofacial database maintained by the Division of Plastic and Reconstructive Surgery. The inclusion criteria consisted of patients with clinical features of RS (glossoptosis, micro- or retrognathia, and respiratory distress, with or without cleft palate) and persistent episodes of respiratory distress, defined as a documented 10% oxygen desaturation sustained for 10 seconds or longer at rest, while sleeping, or during feeding. All patients were evaluated by a multidisciplinary team and considered for operative treatment. The exclusion criteria included patients with respiratory failure due to non-airway causes (e.g., congestive heart failure or renal failure), patients with incomplete records, or those who failed non-surgical management of their airway and received definitive airway management.
Since some patients had more than one admission for airway management, data were recorded and analyzed per admission. Baseline patient factors, hospital factors, and polysomnographic (PSG) results were evaluated. The baseline patient factors consisted of demographics, maternal factors, family history, and the presence of other physical anomalies. Demographic variables included gender, gestational age, birth weight, 1-minute and 5-minute Apgar scores, and the presence of associated syndromes. Maternal factors included a history of alcohol, tobacco, or illicit drug use. Family history was noted, specifically of cleft lip and/or palate as well as of associated syndromes. Physical anomalies included cardiac, pulmonary, central nervous system, and renal anomalies, gastroesophageal reflux disease, and cleft lip and/or palate.
The following hospital-related factors were evaluated: age upon admission, nutritional and respiratory status, laboratory values, and PSG findings. The data review assessed the entire course of hospitalization in conservatively treated patients including weight gain, the level of nutritional support, number of desaturation events, and degree of respiratory support (room air versus oxygen supplementation by nasal cannula, NPA, or intubation). Serial weights were obtained from the initial plastic surgery consultation and then weekly over the first month of conservative management from hospital records or office visits depending upon the patient's length of stay. PSG studies were reviewed and provided a grading for the severity of the obstruction. In patients with multiple PSG results, the PSG findings performed in the setting of highest respiratory support were recorded.
The statistical analysis included the univariate analysis of contingency data using the chi-square test and Fisher's exact test. Continuous factors were analyzed using the unpaired t-test (two-tailed) for parametric data and the Mann-Whitney U test for nonparametric data. P-values <0.05 were considered to indicate statistical significance.
RESULTS
Thirty-two infants met the inclusion criteria. The patient demographics are summarized in Table 1. Nine patients (27%) had a syndromic diagnosis: 4 patients were diagnosed with Stickler syndrome, 4 with velocardiofacial syndrome, and 1 with fetal alcohol syndrome. Twenty-seven patients were found to have a concomitant cleft palate (84%) and one patient had a unilateral complete cleft lip (3%). The mean age upon admission for airway management was 38.1 days, with an average hospitalization of 16.8 days. Including outpatient physician encounters, the average follow-up was 22 months (range, 10–36 months).
A common feature of all patients was the ability to maintain an oxygen saturation level >90% with supplemental oxygen as well as continued weight gain, with or without a nasogastric tube. During their hospitalization, thirty infants (94%) required supplemental oxygenation by nasal cannula (n=19, 59%), NPA (n=1, 3%), or intubation (n=10, 31%). Nine infants remained intubated for an average period of 4 days (range, 2–7 days) while 1 infant was successfully extubated within 28 hours. PSG was performed in 26 patients to objectively quantify the degree of respiratory obstruction. The average apnea-hypoxia index (AHI) was 19.2±5.3 events/hour with a range of 4–39 events/hour, and 4% of sleep time was spent with a saturated oxygen level <90%.
Twenty-seven patients (84%) required nutritional support to maintain weight gain. Twenty-four infants (75%) received a nasogastric tube for an average of 11 days, while 3 infants (9%) required continued nutritional support for 2 weeks following nasogastric tube placement, and a gastrostomy tube was placed. Ultimately, all patients demonstrated adequate weight gain and age-adjusted weight percentiles over the first month of conservative intervention (Fig. 1). Absolute weights decreased over the first week by an average of 73 g; however, in subsequent weeks, the infants displayed weight gains of 150–300 g/week. Absolute weights remained on average above the 25th percentile (range, 20th–37th percentile) over the first two weeks following conservative treatment, with a mean weight at the 32nd percentile by 4 weeks (range, 26th–37th percentile).
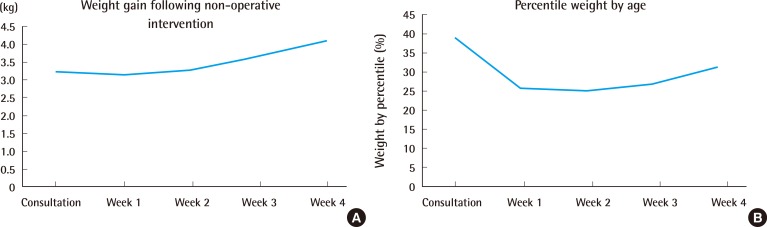
Weight gain
(A) Weight gain average weight gain in kilograms each week following non-operative airway management. All patients demonstrated adequate weight gain and (B) age-adjusted weight percentiles over the first month of conservative interventions.
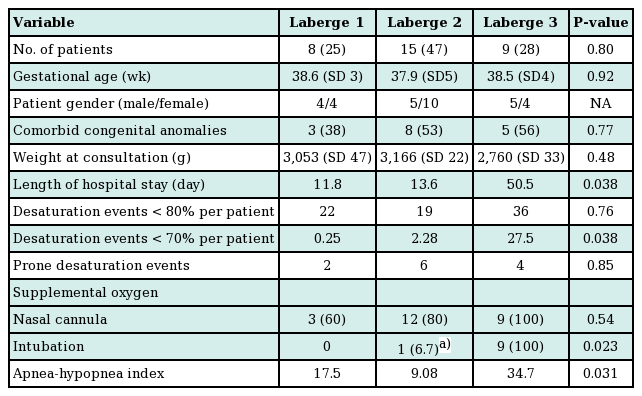
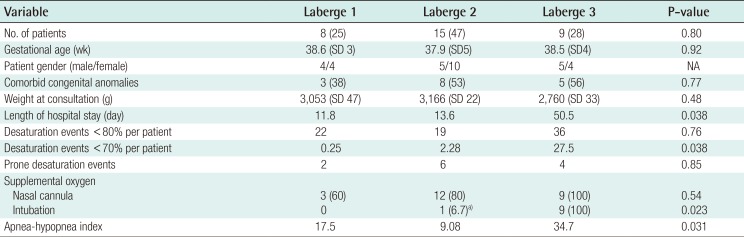
Table 2 summarizes the clinical findings by grade of RS as previously described by Caouette-Laberge et al. [25]., with grades assigned according to whether airway obstruction is relieved by (1) prone positioning, (2) prone positioning and gavage, or (3) endotracheal intubation and gavage. No significant differences were found among demographic factors for infants of different classification grades. Similarly, infant weights were not different according to birth weights, the time of the plastic surgery consultation, hospital discharge, or 1 month following the initiation of conservative measures (Fig. 2). Weight gain over the first 2 weeks following initiation of prone positioning, gavage, and/or intubation demonstrated was greatest in infants classified as grade 3 (8% of body weight), compared to grade 1 (2% of body weight) or grade 2 (3% of body weight) (P<0.05). Additionally, infants with grade 3 RS were found to have a greater AHI, more sleep time with a saturated oxygen level <70% by PSG prior to oxygen supplementation, and a longer hospital course than other infants (Table 2).
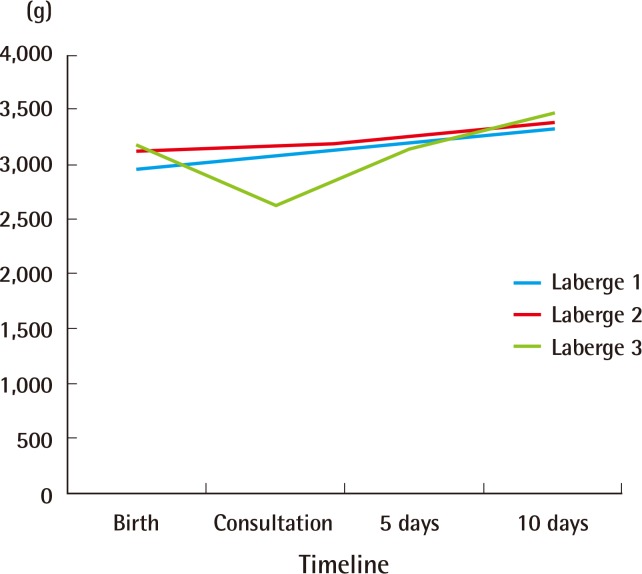
Non-operative weight gain by Laberge classification
Weight gain by Laberge classification. Average infant weights were not different by Laberge classification at birth, the time of the plastic surgery consultation, hospital discharge, or one month following the initiation of conservative measures. The weight gain over the first two weeks following the initiation of prone positioning, gavage, and/or intubation was greatest for infants classified as grade 3 (8% of body weight) compared to grade 1 (2% of body weight) or grade 2 (3% of body weight) (P<0.05).
DISCUSSION
The current study describes the clinical characteristics of a cohort of consecutive patients with RS whose airway was successfully managed without surgical intervention, with all patients discharged home and found to be doing well in outpatient follow-up evaluations. In the past, airway management at our institution was not based on universal guidelines or protocols, but rather on the input of multiple rotating consultants representing various disciplines. The decision for surgical intervention was generally delayed to allow prolonged trials of conservative therapy (e.g., prone positioning, NPA placement, or nutritional support) as long as patients were not in imminent danger of airway compromise. In examining these patients who actually improved with supportive measures, we found that nonsurgical airway management was successful in patients who remained at least at the 25th percentile of weight and had moderate obstruction on PSG, with a mean AHI of 19.2 events/hour.
While the clinical features of the patients set forth in this study provide some guidelines for considering nonoperative care, they are not absolute and must be considered in light of the prevailing clinical, financial, and social context. Many facilities no longer consider nonoperative management adequate for any infant with RS and evidence of airway compromise. Presumably, this position is predicated on concerns that the untimely correction of respiratory and/or feeding difficulties can result in serious complications, extended hospitalizations, and significant morbidity and mortality. Potential major complications include hypoxic brain damage, impaired mental development, cor pulmonale, aspiration pneumonia, and/or failure to thrive [16]. Mortality rates for infants with RS may approach 13.6% [21625]. Moreover, the care of infants with RS may require prolonged hospitalization, with averages ranging from 10 to 60 days [6], translating into exorbitant costs of care. We found that our patients with grade 3 RS in this study had significantly higher AHI values and longer hospitalization times than other patients. It is certainly possible that these patients may have had a shorter course of hospitalization had surgical intervention been performed earlier in the hospital stay. Evidence also indicates that a delayed surgical airway intervention in patients who eventually require it may be less effective [8].
Our relatively simple criteria for considering the nonoperative treatment of patients with RS is more objective than the largely subjective grading systems proposed by Caouette-Laberge et al. [25] They do share some similarities to the rule-of-thumb criteria for tongue-lip adhesion proposed by Parsons and Smith, which included progressive weight/strength gain over a 7-day period and unsuccessful extubation after 3 days. While outlining general indications for surgical intervention, these criteria were based upon a single surgeon's opinion rather than statistically supported data.
The limitations of this study include its retrospective nature, small study population, and the absence of complete PSG data for 6 cases. In the absence of a universal protocol, not all patients were studied with PSG. Furthermore, the decision to operate or to continue with conservative measures was based on the input of multiple consulting teams rather than a rigorous set of reproducible clinical factors. Given the retrospective nature of this study, it is impossible to predict the success of conservative treatment for patients with RS whose airway may or may not require surgical intervention. In addition, this study does not directly address the issue of which infants with RS require surgical airway intervention, which is the focus of an ongoing study. The characteristics of patients with RS who were successfully managed with conservative measures alone may not necessarily correspond to the characteristics of patients who require surgical airway intervention.
This retrospective study of infants with severe RS admitted to the neonatal intensive care unit of a single institution documented that weight gain and mild to moderate obstruction on PSG was associated with successful conservative airway management and safe discharge to home. While some patients with RS will clearly do well with conservative measures and other patients with RS obviously require surgical airway procedures, the decision to operate or not to operate may be difficult in a significant proportion of patients with RS. For these patients, the clinical characteristics identified in the current study may help avoid unnecessary surgical airway interventions. An ongoing study to compare these patients with infants who underwent surgical airway procedures is being completed at our institution and will be reported in the near future.
Notes
No potential conflict of interest relevant to this article was reported.
This article was presented at the 31st annual meeting Northeastern Society of Plastic Surgeons on September 12-14, 2014 in Providence, RI, USA.